

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019010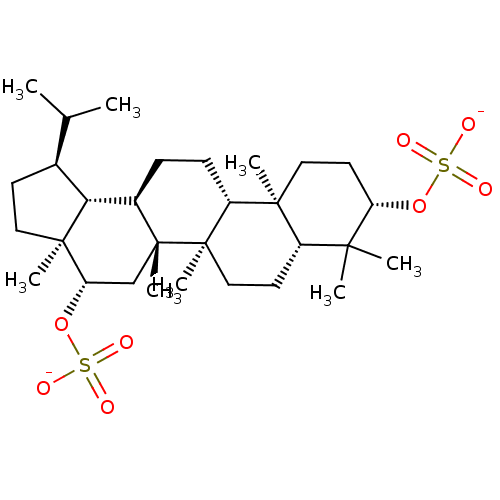 (CHEMBL3288082) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Uncompetitive inhibition of Pacific electric ray AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554708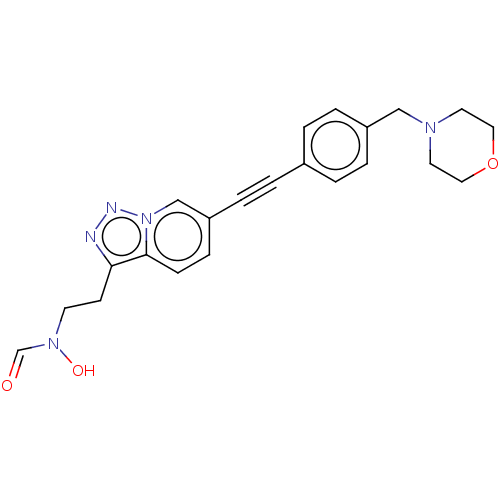 (CHEMBL4746493) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554707 (CHEMBL4751248) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554702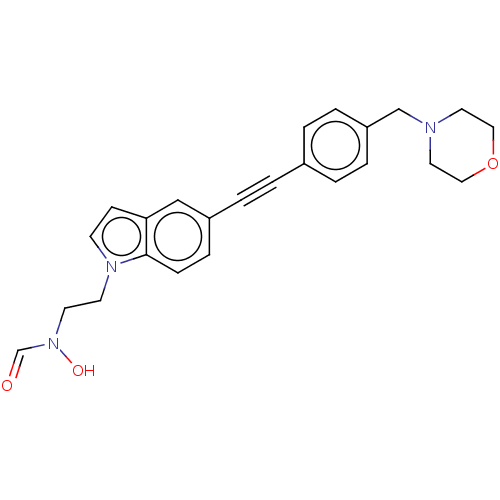 (CHEMBL4776055) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554703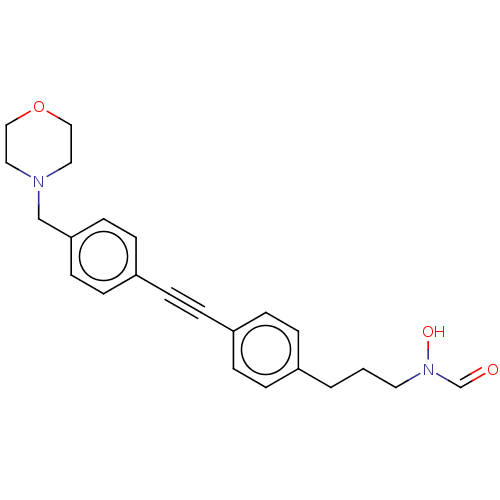 (CHEMBL4798753) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554700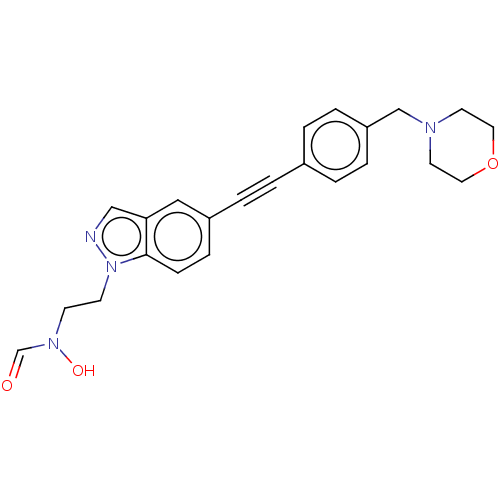 (CHEMBL4786538) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554704 (CHEMBL4755244) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554709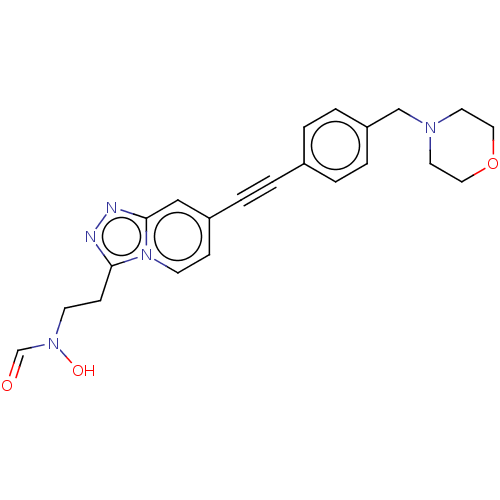 (CHEMBL4755307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554717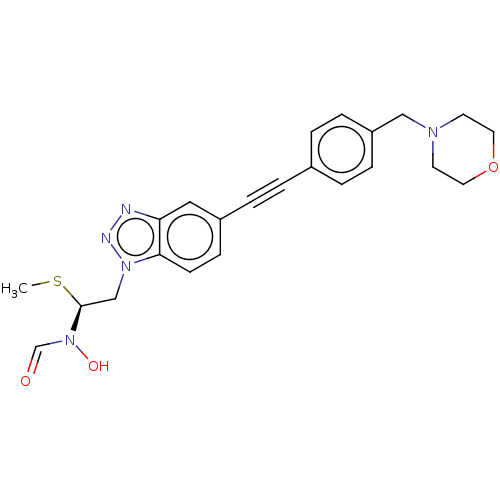 (CHEMBL4746231) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554725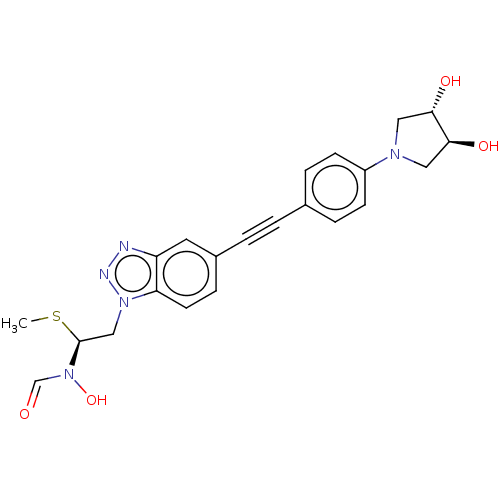 (CHEMBL4780225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554705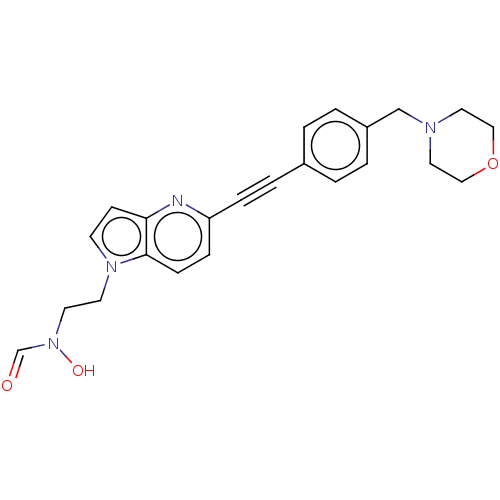 (CHEMBL4796789) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554706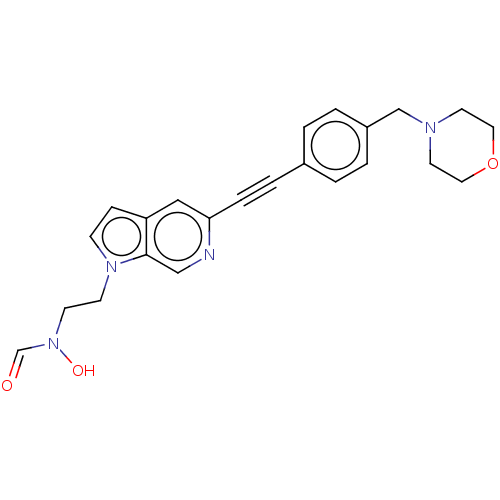 (CHEMBL4761278) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554724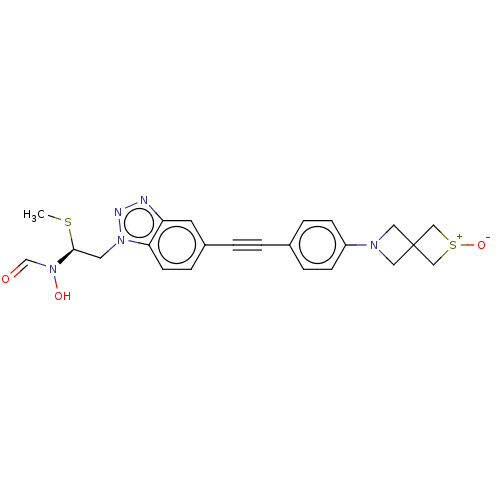 (CHEMBL4786712) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554723 (CHEMBL4758871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554722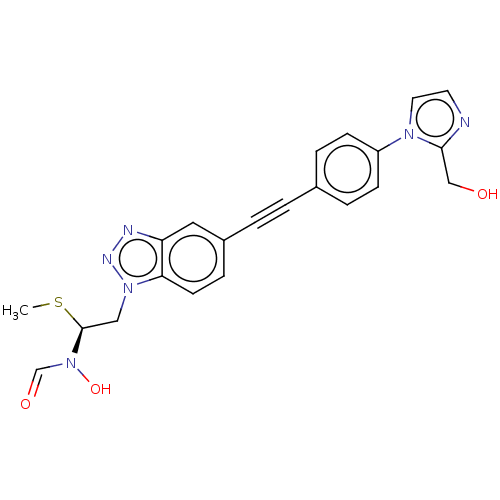 (CHEMBL4776397) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554701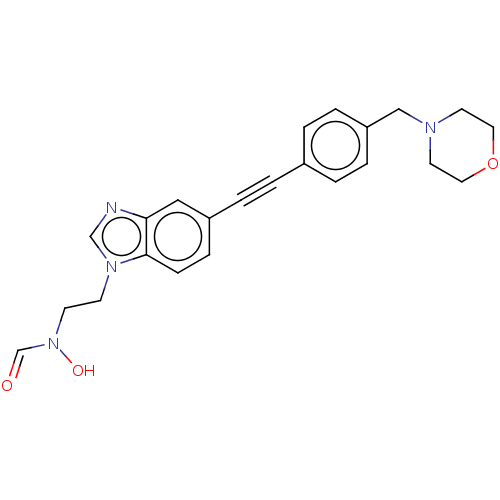 (CHEMBL4760055) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554713 (CHEMBL4794466) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554712 (CHEMBL4779531) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50554717 (CHEMBL4746231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AchE expressed in HEK293 cells using acetylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554714 (CHEMBL4741753) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554715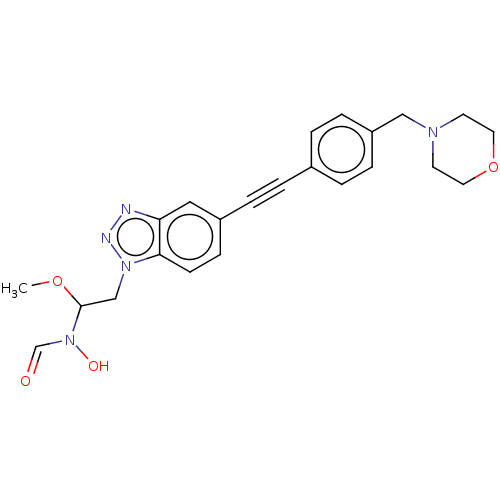 (CHEMBL4753921) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554720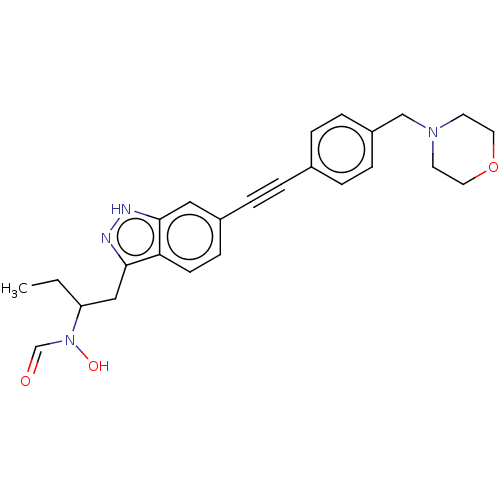 (CHEMBL4756364) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554711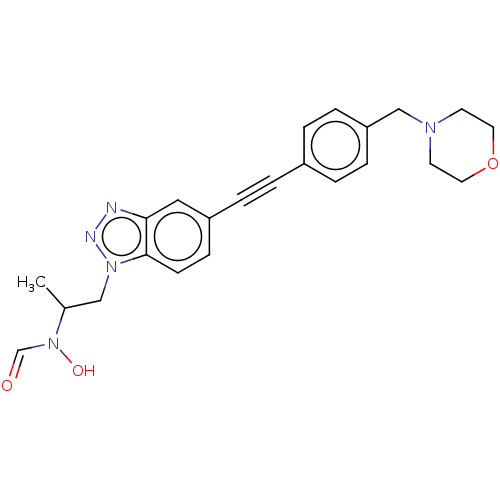 (CHEMBL4794968) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50554722 (CHEMBL4776397) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AchE expressed in HEK293 cells using acetylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554718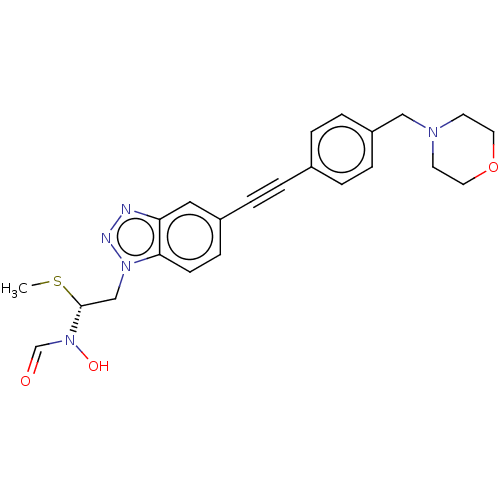 (CHEMBL4763862) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019013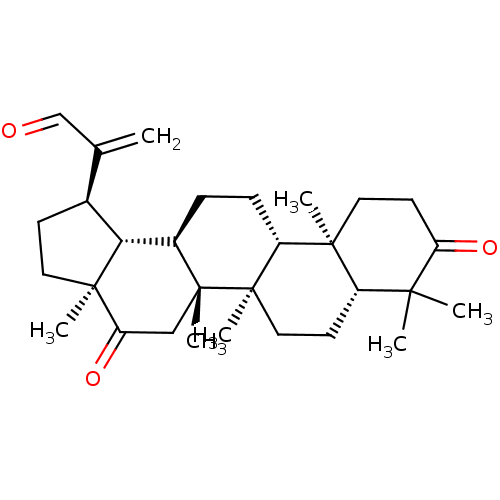 (CHEMBL3288094) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50554725 (CHEMBL4780225) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AchE expressed in HEK293 cells using acetylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554721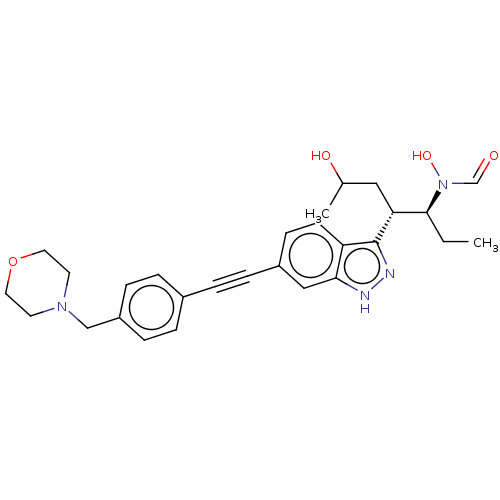 (CHEMBL4756745) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50554723 (CHEMBL4758871) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AchE expressed in HEK293 cells using acetylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554716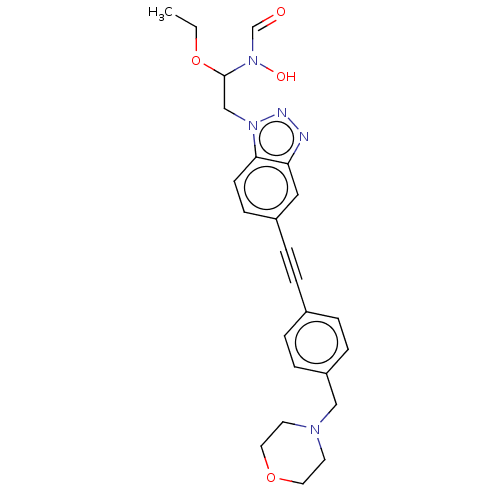 (CHEMBL4797132) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019010 (CHEMBL3288082) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554710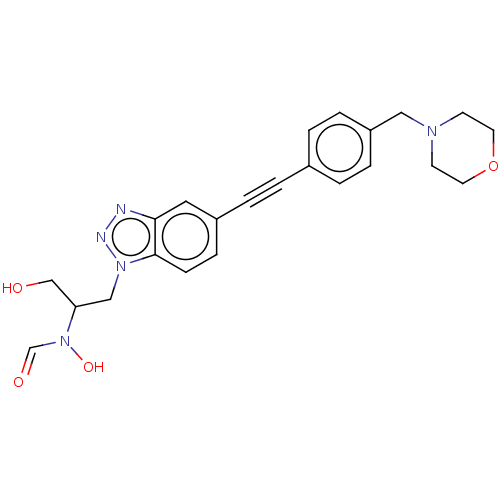 (CHEMBL4797709) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50554719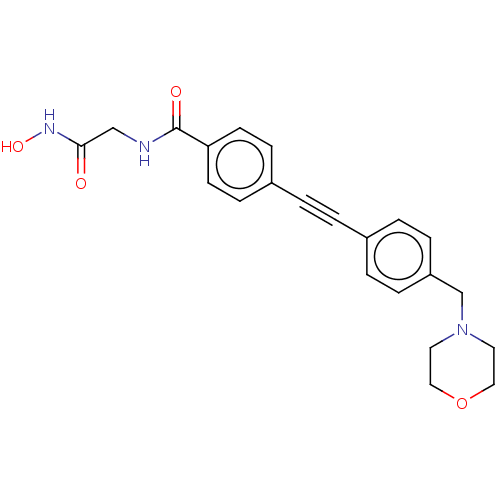 (CHEMBL4780842) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019003 (CHEMBL3288074) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019012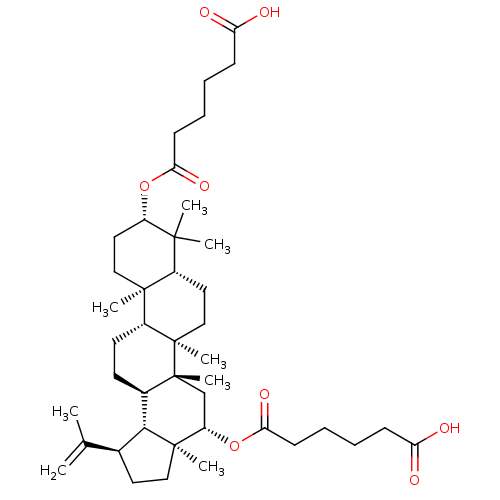 (CHEMBL3288092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50554724 (CHEMBL4786712) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AchE expressed in HEK293 cells using acetylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019010 (CHEMBL3288082) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019009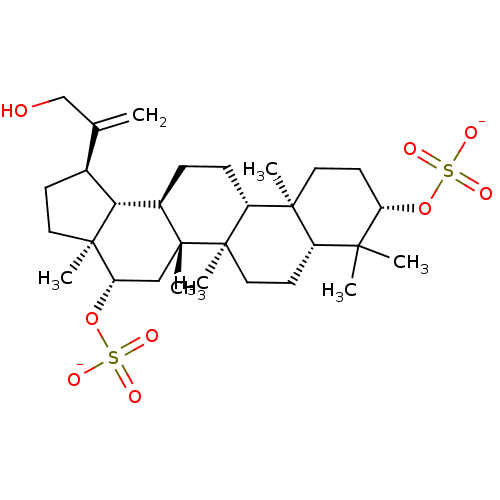 (CHEMBL3288081) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019003 (CHEMBL3288074) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019008 (CHEMBL3288080) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019002 (Calenduladiol) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019013 (CHEMBL3288094) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019012 (CHEMBL3288092) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019011 (CHEMBL3288083) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019009 (CHEMBL3288081) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |