

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156774 (CHEMBL3792888) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156788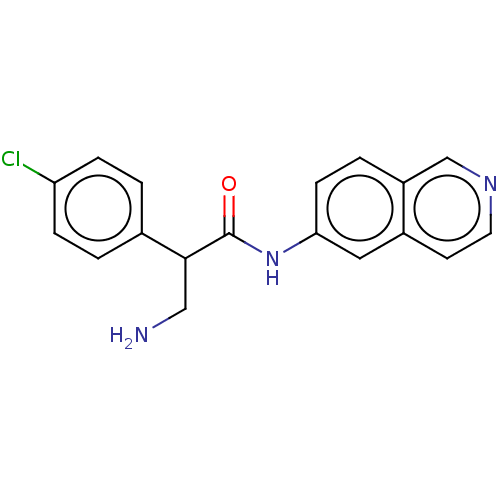 (CHEMBL3794104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156784 (CHEMBL3792673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156799 (CHEMBL3793004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156782 (CHEMBL3792663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156683 (CHEMBL3793788) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156732 (CHEMBL3793497) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50079412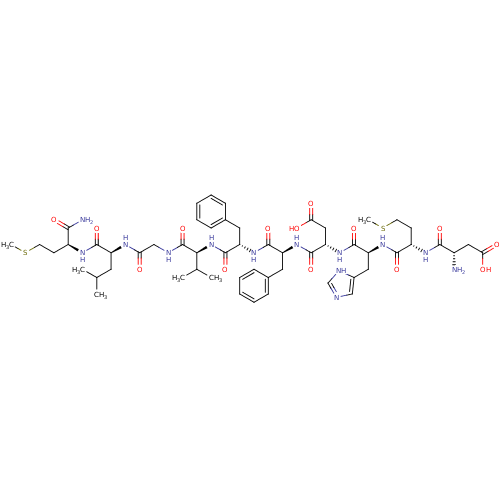 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [125I][MePhe7]NKB from human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156687 (CHEMBL3793029) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50148579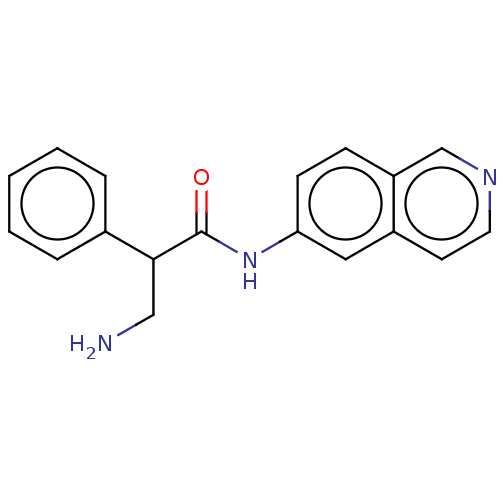 (CHEMBL3770836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156787 (CHEMBL3793404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156685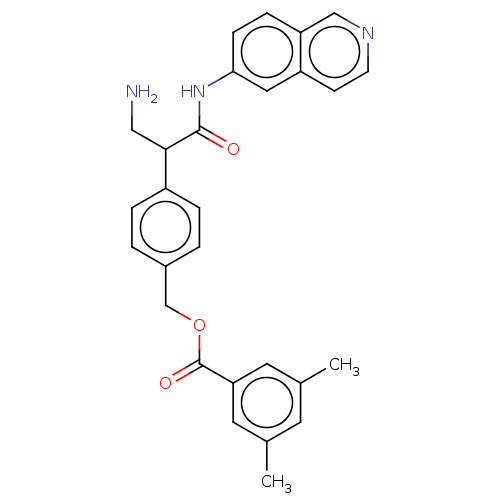 (CHEMBL3792412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156789 (CHEMBL3793505) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156795 (CHEMBL3794270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156793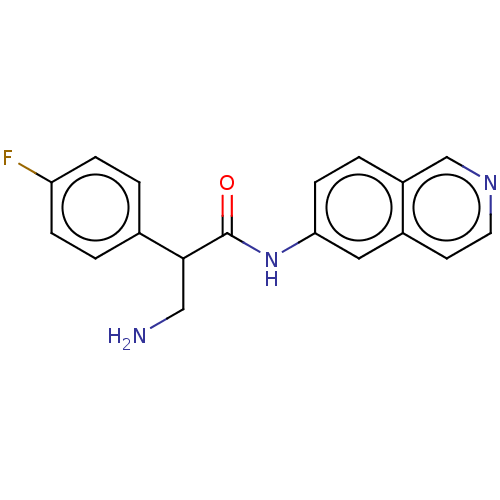 (CHEMBL3792921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156794 (CHEMBL3792666) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156786 (CHEMBL3793489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156691 (CHEMBL3792435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156733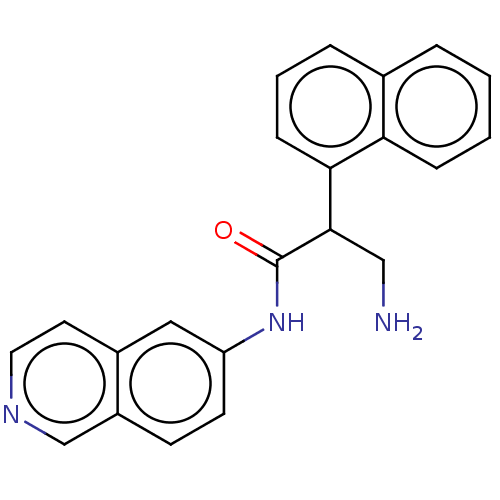 (CHEMBL3793296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156780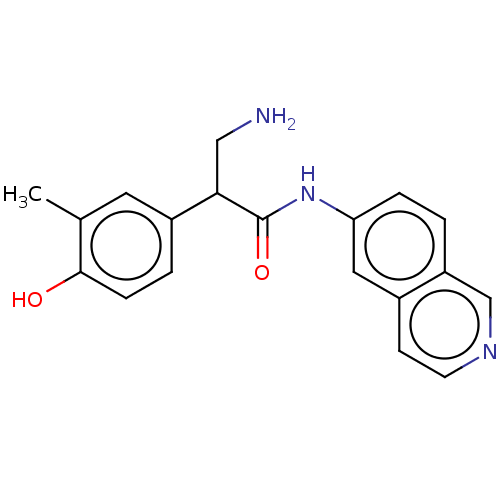 (CHEMBL3794298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156791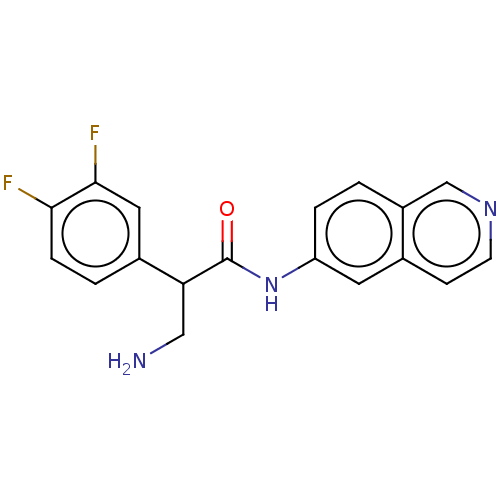 (CHEMBL3793969) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156777 (CHEMBL3793516) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156783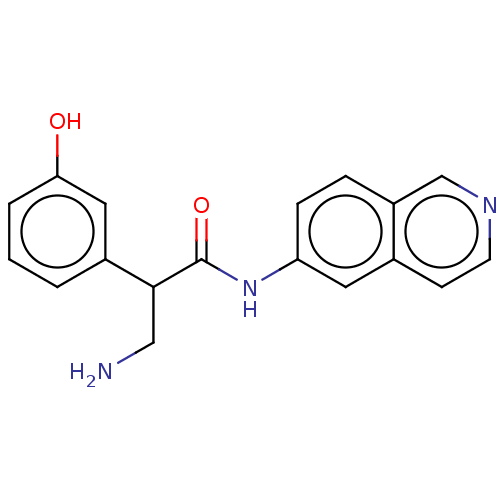 (CHEMBL3792734) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156790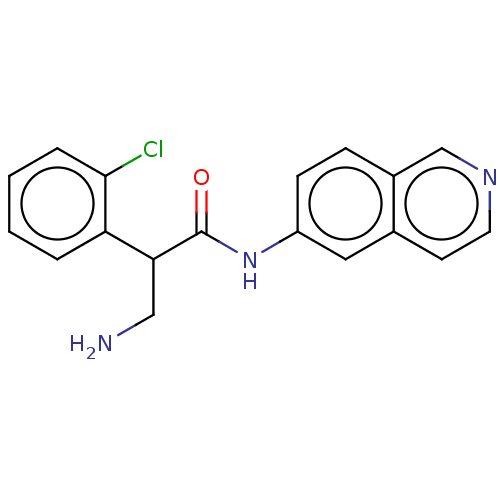 (CHEMBL3792549) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156797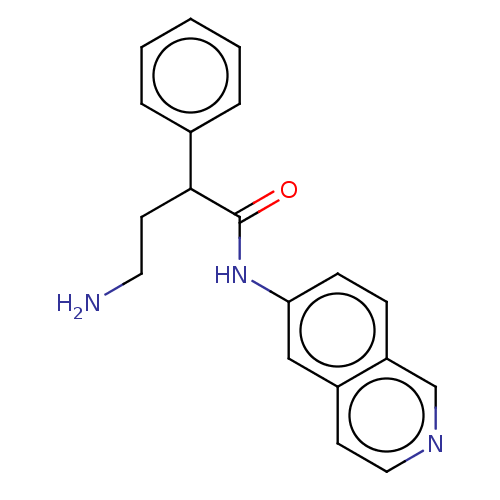 (CHEMBL3793538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50148578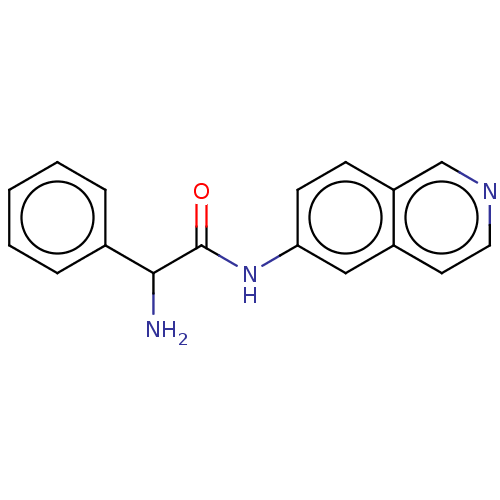 (CHEMBL3770730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50291261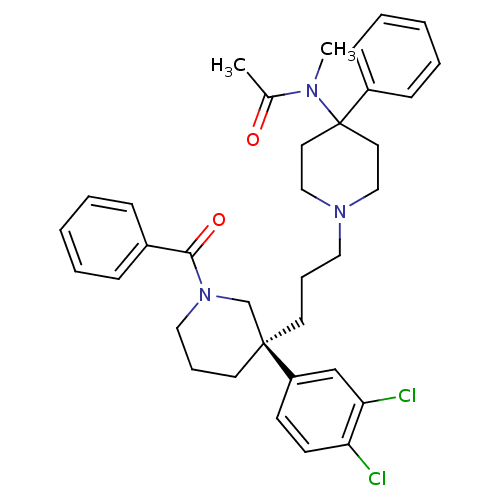 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156684 (CHEMBL3793688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156688 (CHEMBL3792969) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156734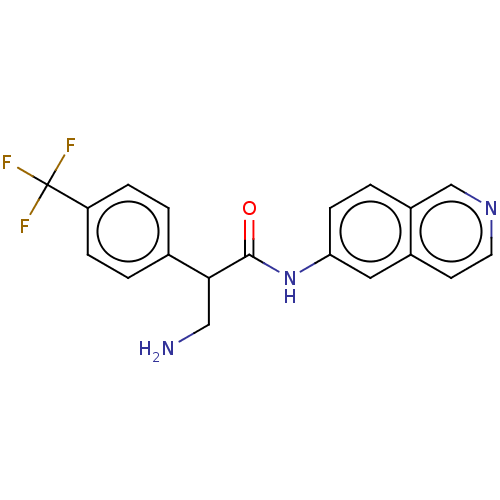 (CHEMBL3793235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156711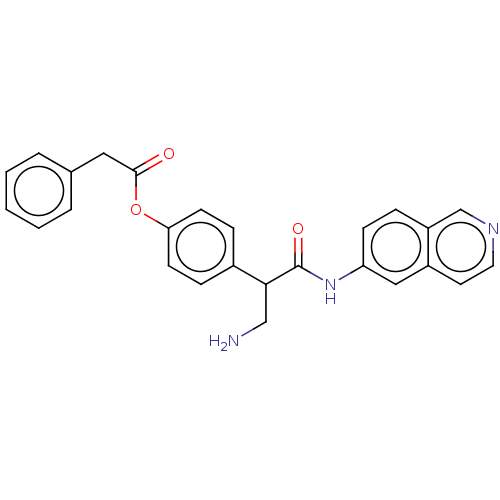 (CHEMBL3792599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50291261 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Binding affinity to human NK3 receptor by radioligand binding assay | Bioorg Med Chem Lett 21: 1991-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.033 BindingDB Entry DOI: 10.7270/Q2B56K16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081194 (CHEMBL3422009 | US10544150, Compound 156) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156776 (CHEMBL3792593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156792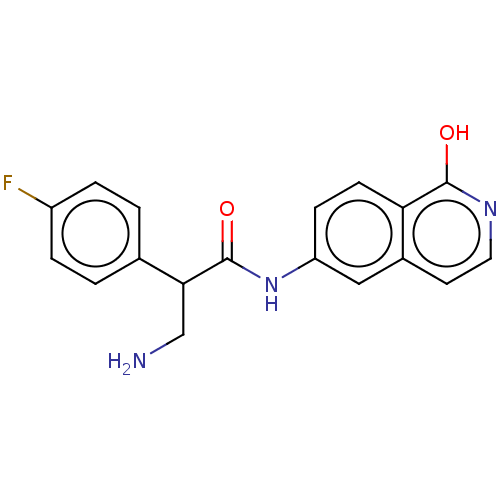 (CHEMBL3792581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156778 (CHEMBL3793153) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156796 (CHEMBL3792781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50319005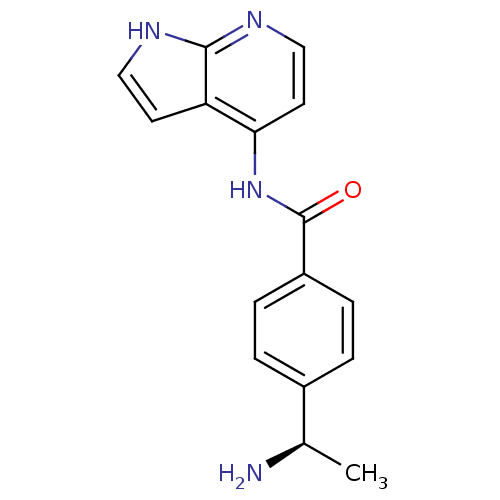 ((R)-4-(1-aminoethyl)-N-(1H-pyrrolo[2,3-b]pyridin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081396 (CHEMBL3422014) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156735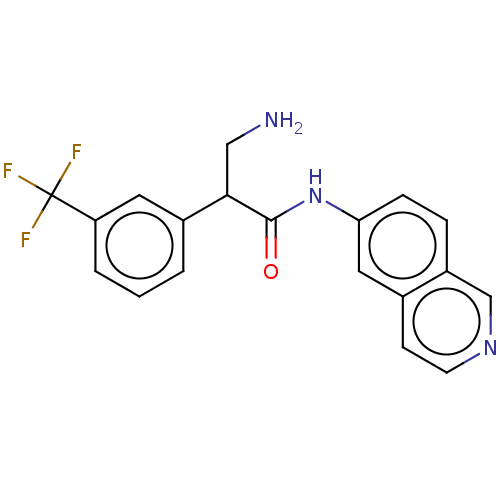 (CHEMBL3793203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178095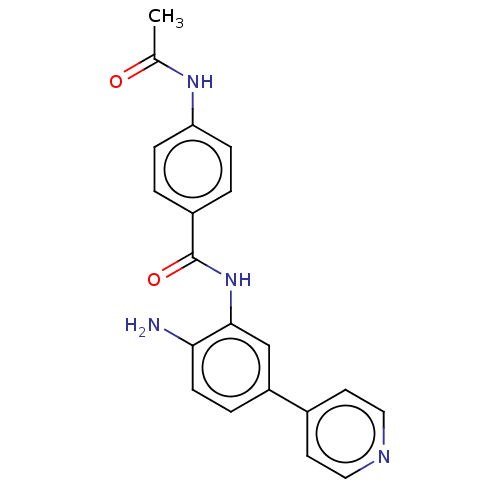 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923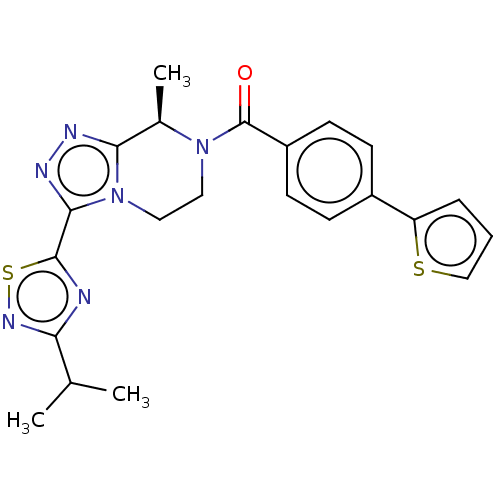 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50156775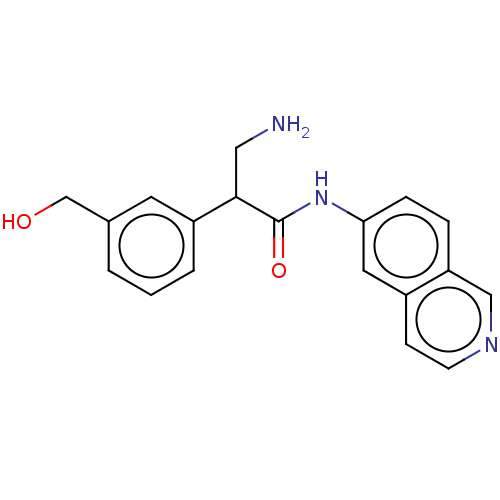 (CHEMBL3792689) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay | Bioorg Med Chem Lett 26: 2475-80 (2016) Article DOI: 10.1016/j.bmcl.2016.03.104 BindingDB Entry DOI: 10.7270/Q2VD71CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196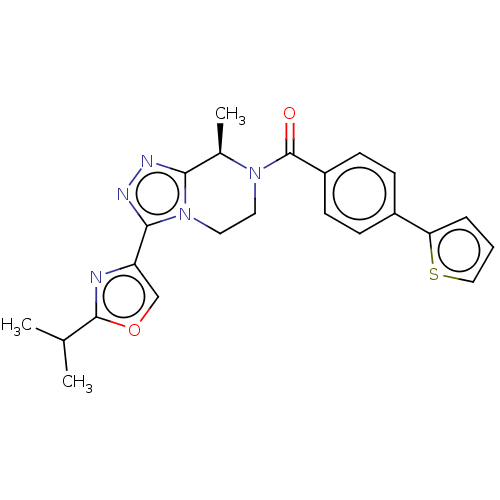 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2259 total ) | Next | Last >> |