

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 10 mins by stopped flow assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241840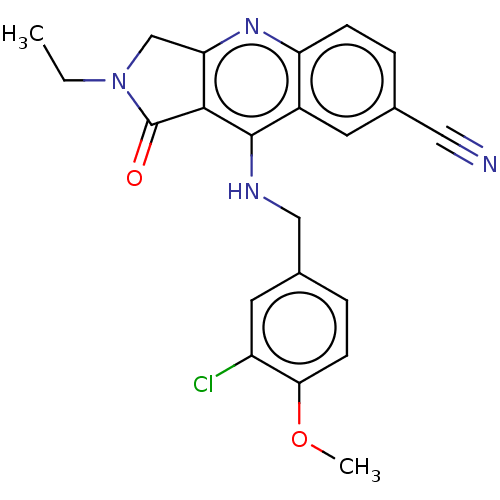 (CHEMBL4072903 | US10899756, Compound K) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50276359 (CHEMBL4129303) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate after 15 mins by amplex red reagent based assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241832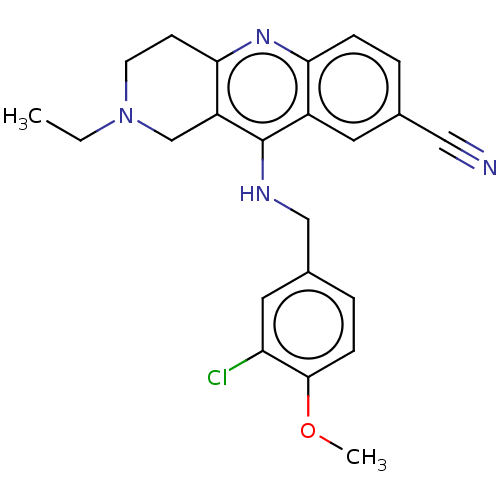 (CHEMBL4083986 | US10626113, Compound C | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428976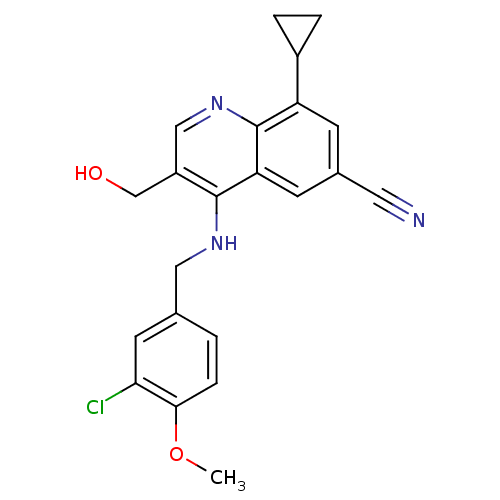 (CHEMBL2333219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.277 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241842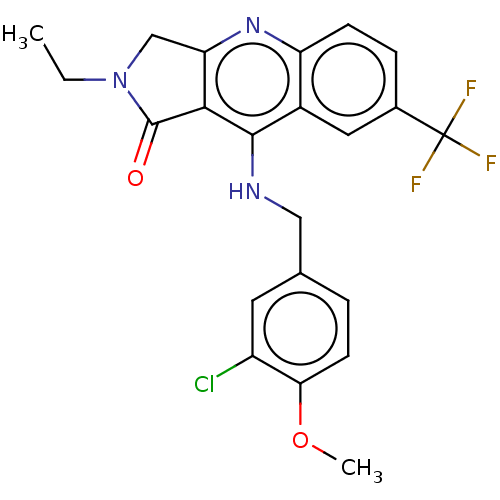 (CHEMBL4064315 | US10899756, Compound M) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of adenylate cyclase via Adenosine A1 receptor in rat fat cell membranes | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241835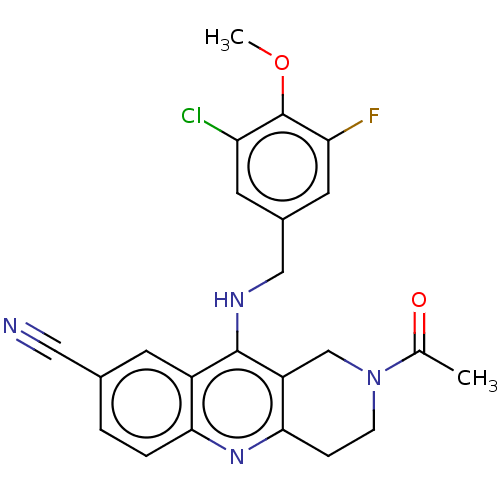 (CHEMBL4092717 | US10899756, Compound AC) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428975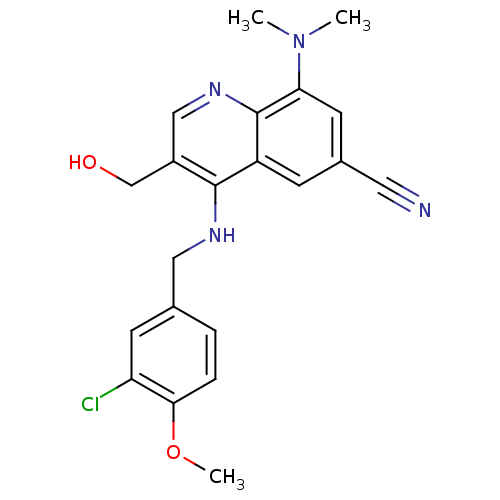 (CHEMBL2333220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50242157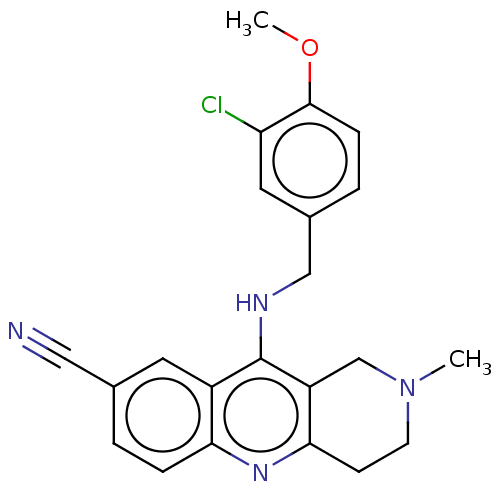 (CHEMBL4070894 | US10626113, Compound B | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428971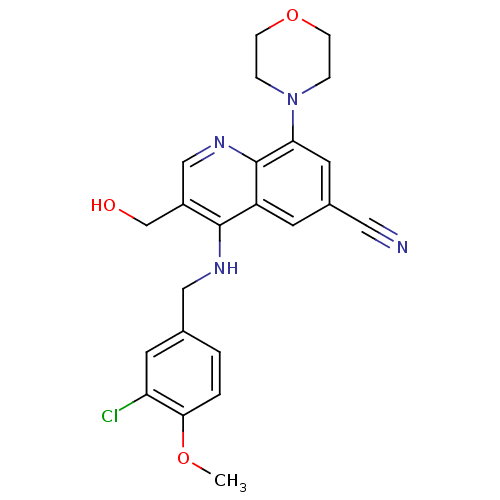 (CHEMBL2333224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241834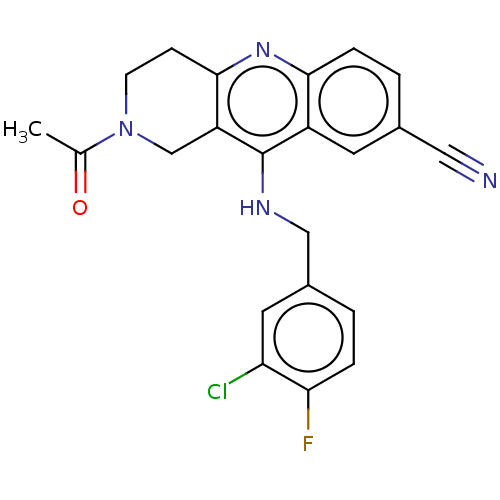 (CHEMBL4065036 | US10626113, Compound J | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241837 (CHEMBL4102913 | US10626113, Compound G | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241843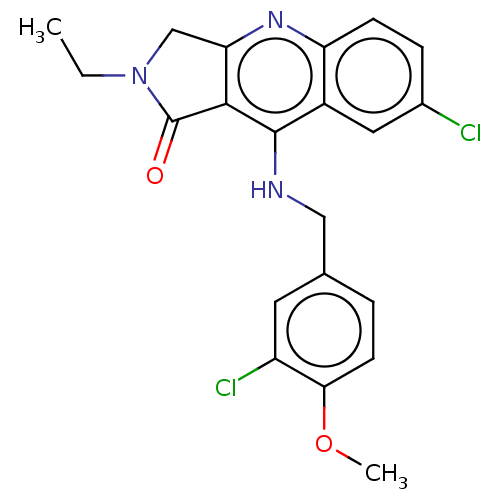 (CHEMBL4102250 | US10899756, Compound N) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241839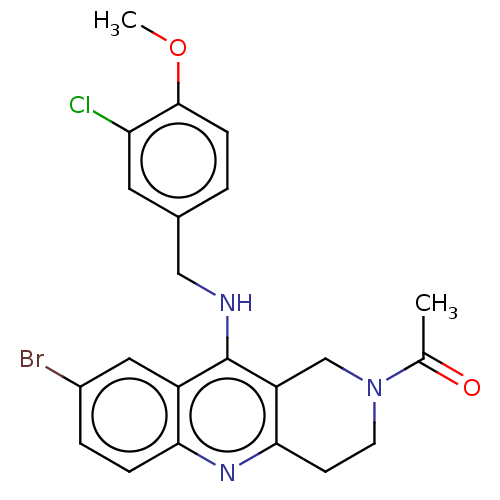 (CHEMBL4083346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241841 (CHEMBL4092040 | US10899756, Compound L) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428972 (CHEMBL2333223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428974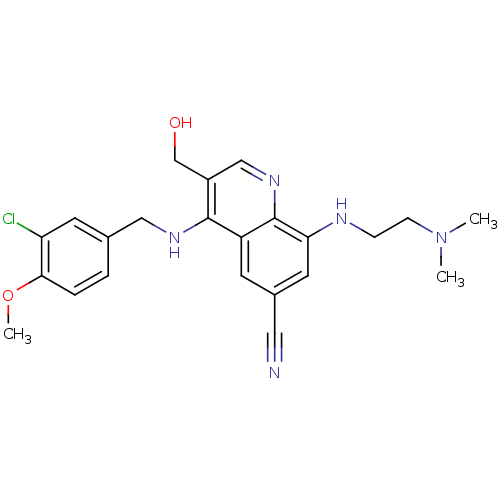 (CHEMBL2333221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50276368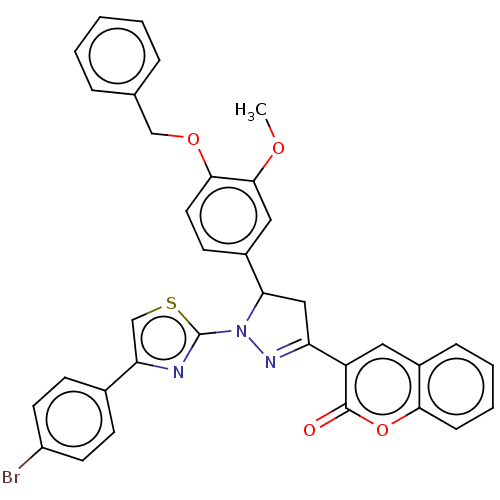 (CHEMBL4128371) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha' (Homo sapiens (Human)) | BDBM50241840 (CHEMBL4072903 | US10899756, Compound K) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428973 (CHEMBL2333222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate after 15 mins by amplex red reagent based assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50242154 (CHEMBL4099788 | US10626113, Compound A | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha' (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE6C using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241838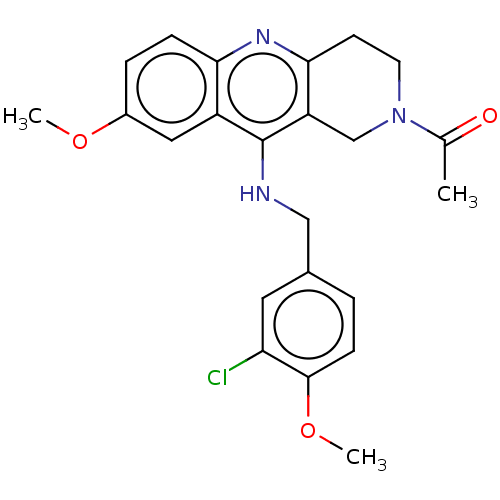 (CHEMBL4061516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50242155 (CHEMBL4100445 | US10899756, Compound AA) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50242158 (CHEMBL4084285 | US10899756, Compound 0) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50242159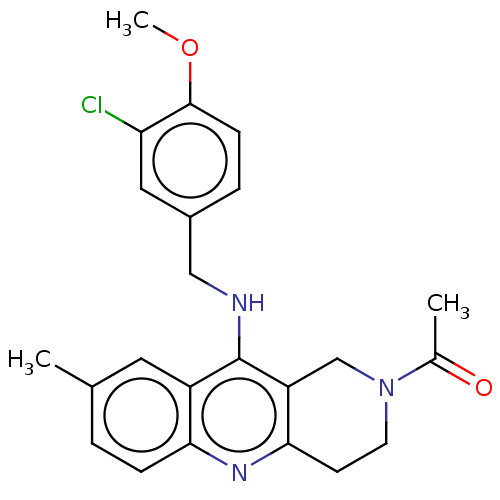 (CHEMBL4084966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of gastric H+/K+ ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193852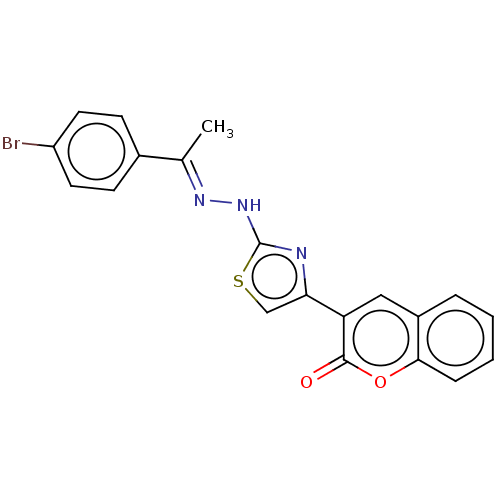 ((E)-3-(2-(2-(1-(4-bromophenyl)ethylidene)hydraziny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276360 (CHEMBL4126559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193866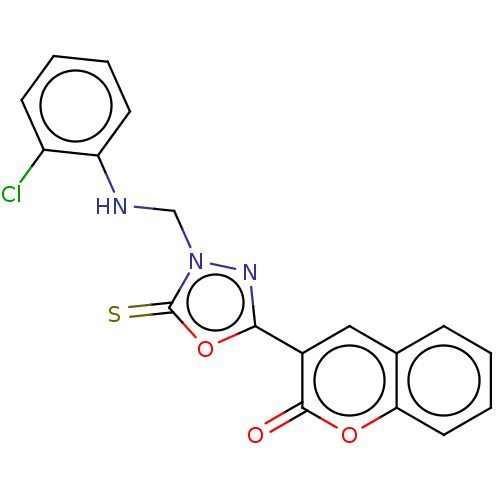 (3-(4-((2-Chlorophenylamino)methyl)-5-thioxo-4,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276366 (CHEMBL4129984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276369 (CHEMBL4127948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276310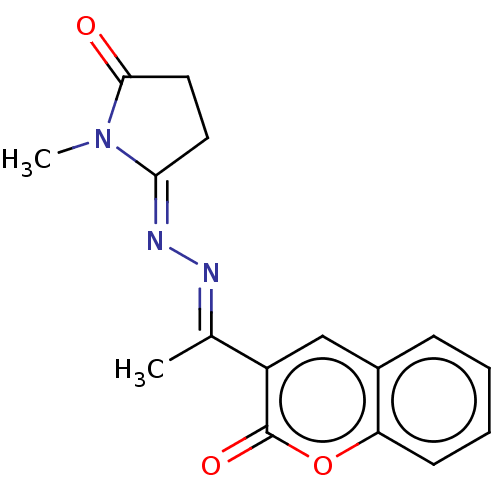 (CHEMBL4127794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50242156 (CHEMBL4096417 | US10899756, Compound P) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241833 (CHEMBL4073617 | US10626113, Compound I | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193869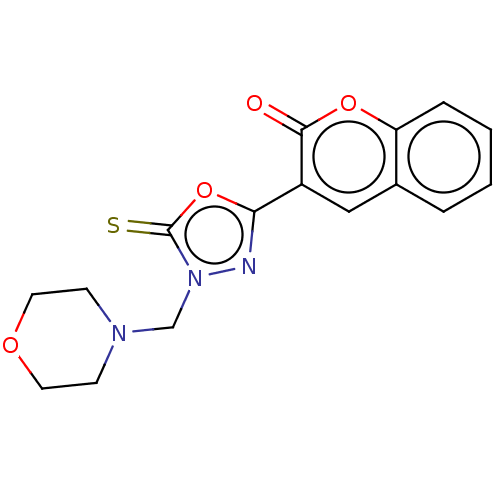 (3-(4-(Morpholinomethyl)-5-thioxo-4,5-dihydro-1,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276367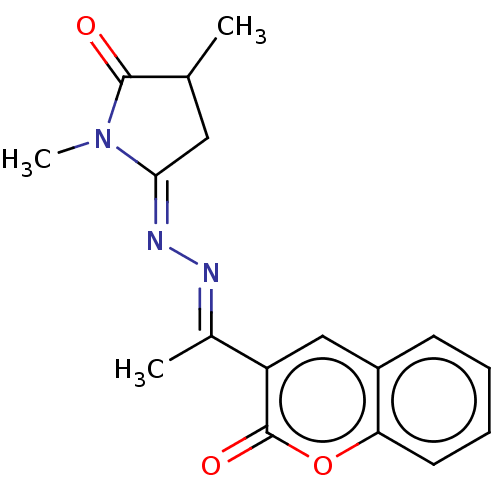 (CHEMBL4129191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193860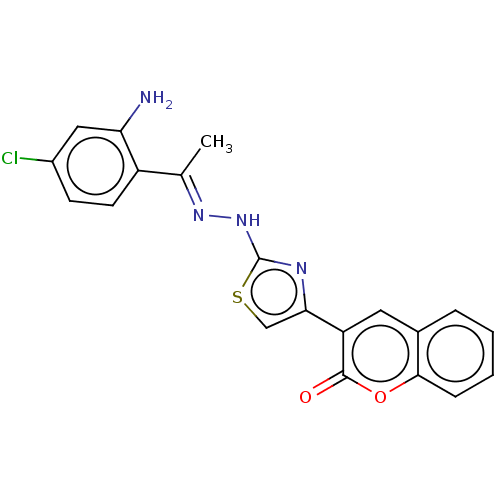 ((E)-3-(2-(2-((2-amino-4-chlorophenyl)(phenyl)methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of bovine kidney ALR1 using D-glucoronic acid as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50276361 (CHEMBL4129493) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf intestinal alkaline phosphatase using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after 30 ... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE2 (unknown origin) using FAM-cGMP and FAM-cAMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193864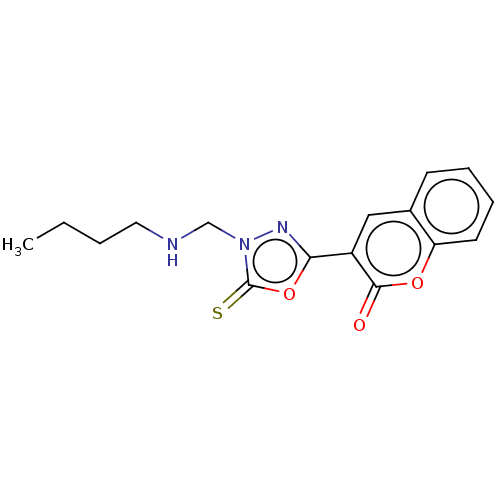 (3-(4-((Butylamino)methyl)-5-thioxo-4,5-dihydro-1,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of bovine kidney ALR1 using D-glucoronic acid as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |