

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50388528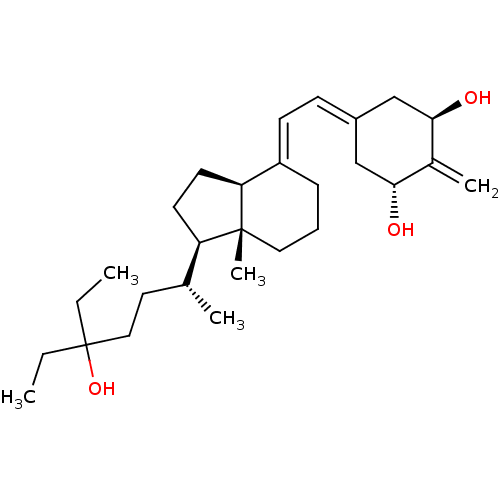 (CHEMBL2058272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-dihydroxyvitamin D3 from human recombinant GST-tagged vitamin D3 receptor LBD expressed in Escherichia coli BL21 after ... | J Med Chem 55: 4373-81 (2012) Article DOI: 10.1021/jm300230a BindingDB Entry DOI: 10.7270/Q25M66R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25-dihydroxyvitamin D3 from recombinant human VDR LBD expressed in Escherichia coli BL21 (DE3) pLysS after 16 hrs | Bioorg Med Chem 23: 7274-81 (2015) Article DOI: 10.1016/j.bmc.2015.10.026 BindingDB Entry DOI: 10.7270/Q2R78H20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50129805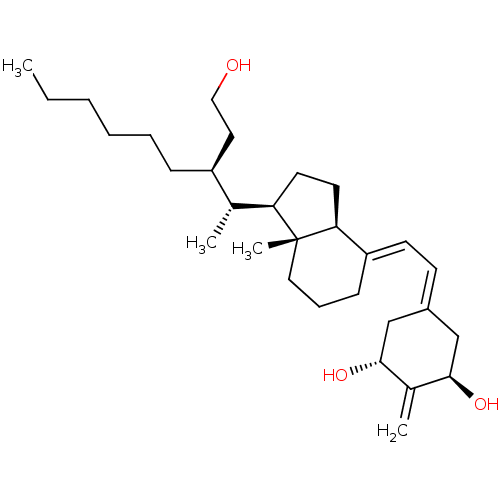 (CHEMBL3629552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25-dihydroxyvitamin D3 from recombinant human VDR LBD expressed in Escherichia coli BL21 (DE3) pLysS after 16 hrs | Bioorg Med Chem 23: 7274-81 (2015) Article DOI: 10.1016/j.bmc.2015.10.026 BindingDB Entry DOI: 10.7270/Q2R78H20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50388529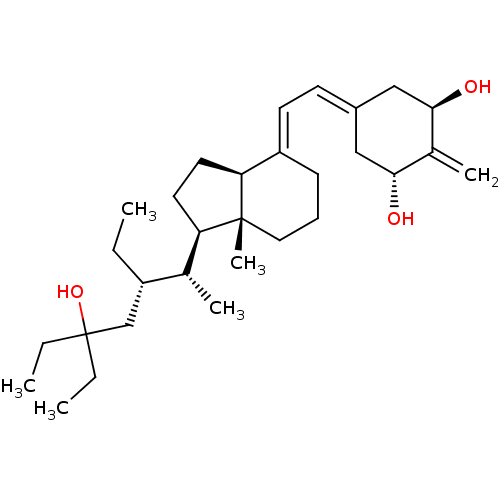 (CHEMBL2058273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-dihydroxyvitamin D3 from human recombinant GST-tagged vitamin D3 receptor LBD expressed in Escherichia coli BL21 after ... | J Med Chem 55: 4373-81 (2012) Article DOI: 10.1021/jm300230a BindingDB Entry DOI: 10.7270/Q25M66R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50388530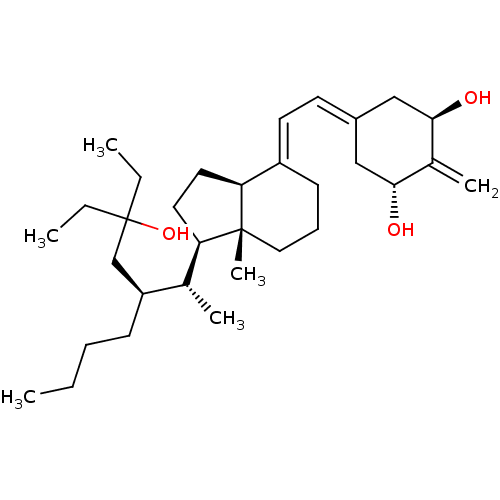 (CHEMBL2058274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-dihydroxyvitamin D3 from human recombinant GST-tagged vitamin D3 receptor LBD expressed in Escherichia coli BL21 after ... | J Med Chem 55: 4373-81 (2012) Article DOI: 10.1021/jm300230a BindingDB Entry DOI: 10.7270/Q25M66R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM159600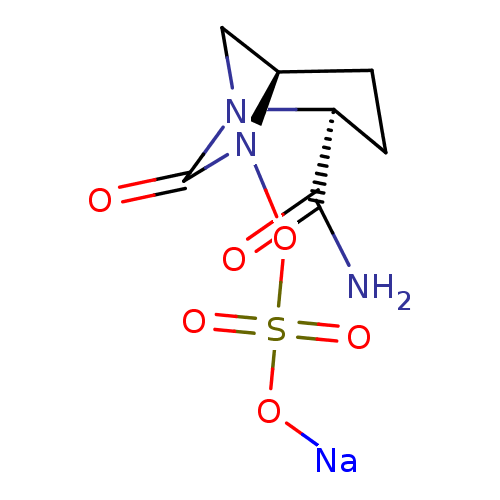 (US8772490, Example 23 | US9035062, 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM212440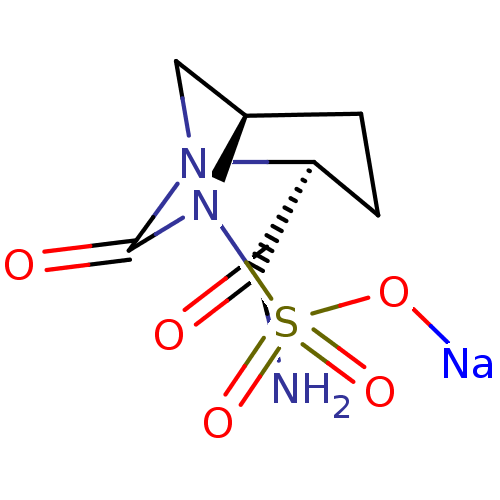 (US8772490, H) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM159600 (US8772490, Example 23 | US9035062, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9035062 (2015) BindingDB Entry DOI: 10.7270/Q2KP80XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM191563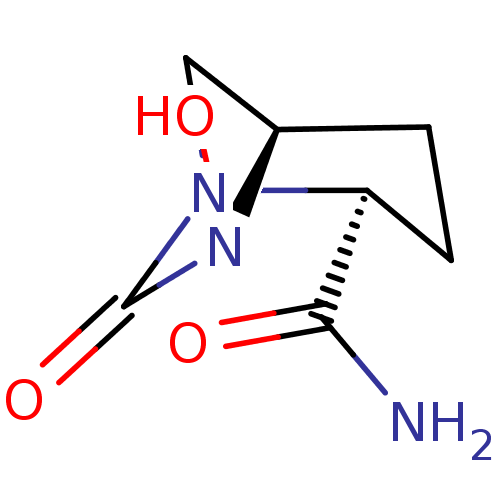 (US9676777, example 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9676777 (2017) BindingDB Entry DOI: 10.7270/Q2G15Z17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM212441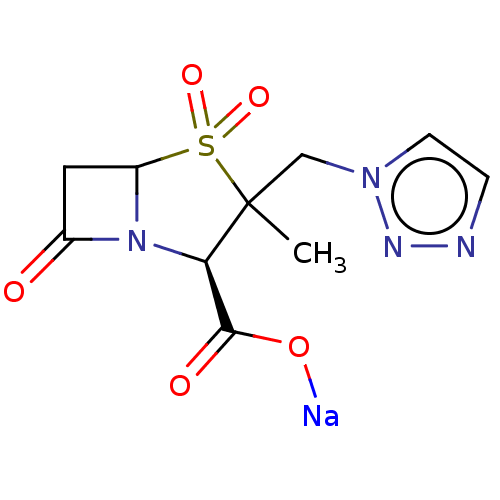 (US8772490, TAZ) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9676777 (2017) BindingDB Entry DOI: 10.7270/Q2G15Z17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50157692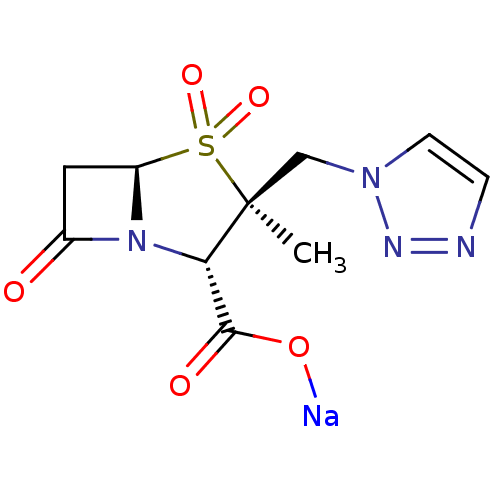 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9035062 (2015) BindingDB Entry DOI: 10.7270/Q2KP80XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM191564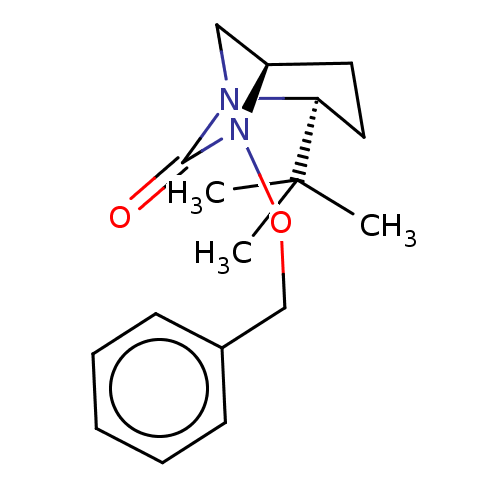 (US9676777, example 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9676777 (2017) BindingDB Entry DOI: 10.7270/Q2G15Z17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM159601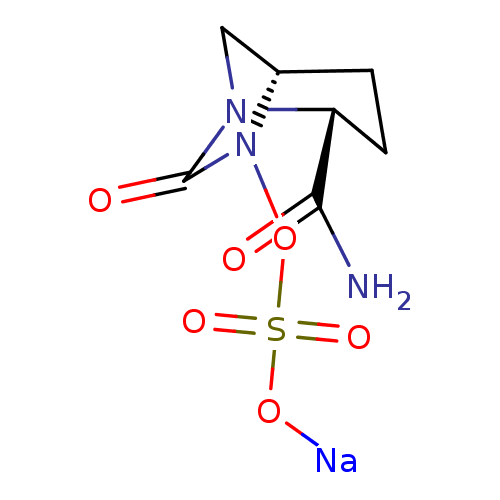 (US8772490, Example 24 | US9035062, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
MEIJI SEIKA PHARMA CO., LTD. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 μM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% D... | US Patent US9035062 (2015) BindingDB Entry DOI: 10.7270/Q2KP80XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM159601 (US8772490, Example 24 | US9035062, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM159601 (US8772490, Example 24 | US9035062, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Meiji Seika Pharma Co., Ltd. US Patent | Assay Description For the measurement of β-lactamase inhibitory activity, 100 uM (final concentration) nitrocefin (Oxoid) was used as a substrate, and 2.5% DMSO, ... | US Patent US8772490 (2014) BindingDB Entry DOI: 10.7270/Q2NK3CWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293099 ((1alpha,22S,24S)-22-Butyl-1,24-dihydroxyvitamin D3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293098 ((1R,3S,5Z)-5-[(2E)-2-{(1R,3aS,7aR)-1-[(1R,2S,4R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293097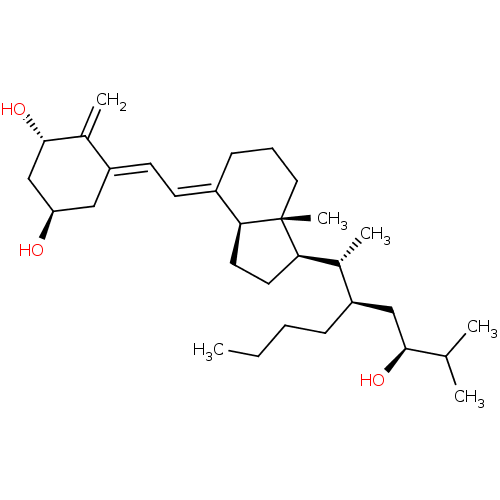 ((1alpha,22R,24S)-22-Butyl-1,24-dihydroxyvitamin D3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293096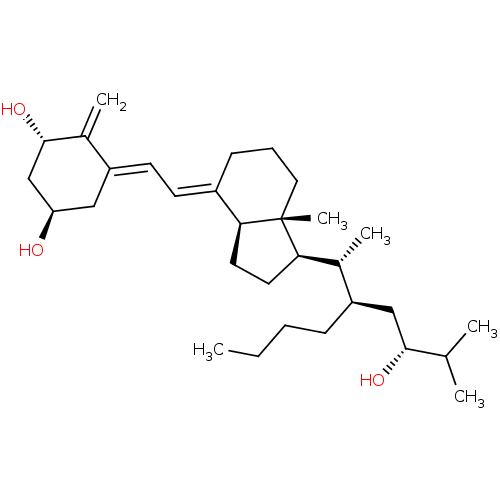 ((1alpha,22R,24R)-22-Butyl-1,24-dihydroxyvitamin D3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293095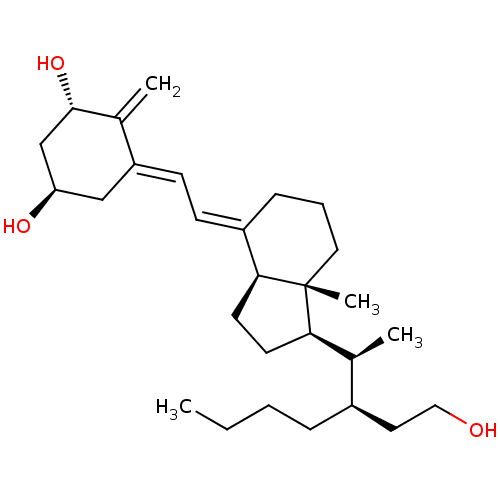 (20S,22R-Butyl-1alpha,24-dihydroxy-24,25,26-trinorv...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293094 ((1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2S,3S)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293093 ((1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2R,3S)-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50293092 (22R-Butyl-1alpha,24-dihydroxy-24,25,26-trinorvitam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Bos taurus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor | J Med Chem 52: 1438-49 (2010) Article DOI: 10.1021/jm8014348 BindingDB Entry DOI: 10.7270/Q2251J6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||