 Found 153 hits with Last Name = 'santucci' and Initial = 'a'
Found 153 hits with Last Name = 'santucci' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264305
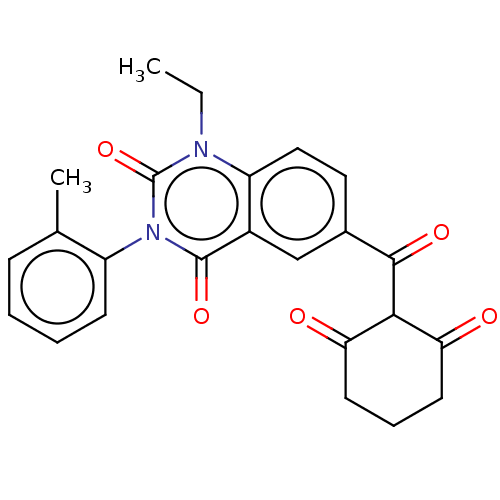
(CHEMBL4102251)Show SMILES CCn1c2ccc(cc2c(=O)n(-c2ccccc2C)c1=O)C(=O)C1C(=O)CCCC1=O |(23.66,-34.72,;25,-33.95,;25,-32.41,;23.68,-31.64,;22.34,-32.41,;21.01,-31.63,;21.02,-30.09,;22.35,-29.33,;23.68,-30.09,;25.02,-29.32,;25.02,-27.78,;26.35,-30.1,;27.69,-29.34,;27.69,-27.81,;29.02,-27.04,;30.36,-27.82,;30.35,-29.36,;29.02,-30.12,;29.01,-31.66,;26.35,-31.65,;27.68,-32.43,;19.69,-29.32,;19.69,-27.78,;18.35,-30.08,;17.02,-29.31,;17.02,-27.77,;15.7,-30.08,;15.7,-31.62,;17.02,-32.39,;18.35,-31.62,;19.69,-32.4,)| Show InChI InChI=1S/C24H22N2O5/c1-3-25-18-12-11-15(22(29)21-19(27)9-6-10-20(21)28)13-16(18)23(30)26(24(25)31)17-8-5-4-7-14(17)2/h4-5,7-8,11-13,21H,3,6,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264309

(CHEMBL4060588)Show SMILES CC1CC(=O)C(C(=O)c2cc3cccc(C)c3nc2C#N)C(=O)C1 |(52.99,-30.01,;54.32,-29.24,;54.32,-27.7,;55.65,-26.92,;55.65,-25.38,;56.98,-27.7,;58.32,-26.93,;58.32,-25.39,;59.65,-27.71,;59.64,-29.25,;60.97,-30.02,;60.97,-31.55,;62.3,-32.32,;63.63,-31.55,;63.63,-30.02,;64.96,-29.24,;62.3,-29.25,;62.31,-27.71,;60.98,-26.94,;60.97,-25.41,;60.97,-23.87,;56.98,-29.24,;58.31,-30.01,;55.65,-30,)| Show InChI InChI=1S/C19H16N2O3/c1-10-6-15(22)17(16(23)7-10)19(24)13-8-12-5-3-4-11(2)18(12)21-14(13)9-20/h3-5,8,10,17H,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264308

(CHEMBL4061385)Show SMILES CC1CC(=O)C(C(=O)c2cc3cccc(C)c3nc2C)C(=O)C1 |(27.92,-30.13,;29.26,-29.36,;29.26,-27.82,;30.58,-27.04,;30.58,-25.5,;31.91,-27.82,;33.25,-27.05,;33.25,-25.51,;34.58,-27.82,;34.57,-29.37,;35.9,-30.14,;35.9,-31.67,;37.23,-32.44,;38.56,-31.66,;38.56,-30.13,;39.89,-29.36,;37.24,-29.37,;37.24,-27.83,;35.91,-27.06,;35.91,-25.52,;31.91,-29.36,;33.25,-30.13,;30.58,-30.12,)| Show InChI InChI=1S/C19H19NO3/c1-10-7-15(21)17(16(22)8-10)19(23)14-9-13-6-4-5-11(2)18(13)20-12(14)3/h4-6,9-10,17H,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264307
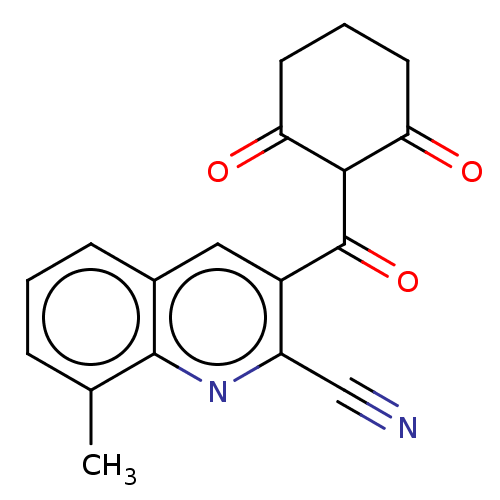
(CHEMBL4093474)Show InChI InChI=1S/C18H14N2O3/c1-10-4-2-5-11-8-12(13(9-19)20-17(10)11)18(23)16-14(21)6-3-7-15(16)22/h2,4-5,8,16H,3,6-7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264306
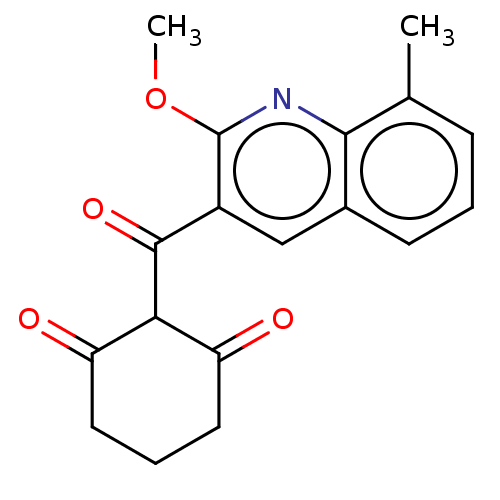
(CHEMBL4071950)Show InChI InChI=1S/C18H17NO4/c1-10-5-3-6-11-9-12(18(23-2)19-16(10)11)17(22)15-13(20)7-4-8-14(15)21/h3,5-6,9,15H,4,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264350

(CHEMBL4080249)Show InChI InChI=1S/C18H17NO3/c1-10-5-3-6-12-9-13(11(2)19-17(10)12)18(22)16-14(20)7-4-8-15(16)21/h3,5-6,9,16H,4,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264374
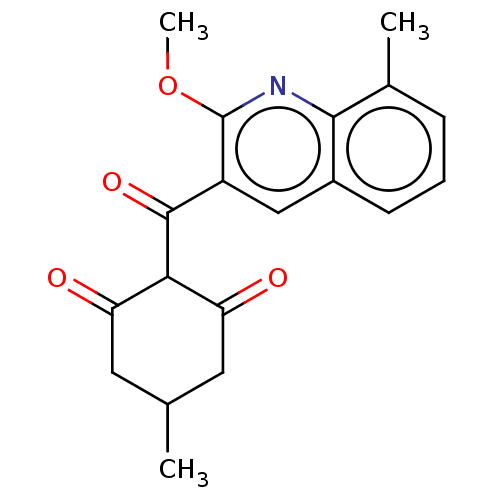
(CHEMBL4069617)Show SMILES COc1nc2c(C)cccc2cc1C(=O)C1C(=O)CC(C)CC1=O |(47.5,-24.58,;48.84,-25.35,;48.84,-26.89,;50.17,-27.65,;50.16,-29.2,;51.49,-29.96,;52.82,-29.19,;51.49,-31.49,;50.16,-32.27,;48.83,-31.5,;48.83,-29.96,;47.5,-29.19,;47.51,-27.65,;46.18,-26.88,;46.18,-25.34,;44.84,-27.64,;43.51,-26.87,;43.51,-25.33,;42.18,-27.64,;42.18,-29.18,;40.85,-29.96,;43.51,-29.95,;44.84,-29.18,;46.17,-29.96,)| Show InChI InChI=1S/C19H19NO4/c1-10-7-14(21)16(15(22)8-10)18(23)13-9-12-6-4-5-11(2)17(12)20-19(13)24-3/h4-6,9-10,16H,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264336

(TEMBOTRIONE)Show SMILES CS(=O)(=O)c1ccc(C(=O)C2C(=O)CCCC2=O)c(Cl)c1COCC(F)(F)F Show InChI InChI=1S/C17H16ClF3O6S/c1-28(25,26)13-6-5-9(15(18)10(13)7-27-8-17(19,20)21)16(24)14-11(22)3-2-4-12(14)23/h5-6,14H,2-4,7-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli JM109 using HPPA as substrate after 10 mins |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264337
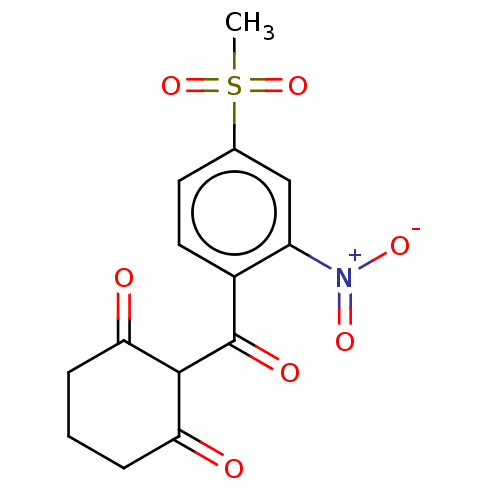
(CHEBI:38321 | MESOTRIONE)Show SMILES CS(=O)(=O)c1ccc(C(=O)C2C(=O)CCCC2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C14H13NO7S/c1-23(21,22)8-5-6-9(10(7-8)15(19)20)14(18)13-11(16)3-2-4-12(13)17/h5-7,13H,2-4H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli BL21(DE3) using HPPA as substrate after 10 mins by UV-Vis spectrometric analysi... |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264333

(CHEMBL4082475)Show InChI InChI=1S/C15H16O4/c1-10-5-2-3-8-14(10)19-9-13(18)15-11(16)6-4-7-12(15)17/h2-3,5,8,15H,4,6-7,9H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli JM109 using HPPA as substrate after 10 mins |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264377

(CHEMBL4069274)Show InChI InChI=1S/C14H13ClO4/c15-9-4-6-10(7-5-9)19-8-13(18)14-11(16)2-1-3-12(14)17/h4-7,14H,1-3,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli JM109 using HPPA as substrate after 10 mins |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264335

(CHEMBL4080003)Show SMILES [O-][N+](=O)c1ccc(OCC(=O)C2C(=O)CCCC2=O)cc1 Show InChI InChI=1S/C14H13NO6/c16-11-2-1-3-12(17)14(11)13(18)8-21-10-6-4-9(5-7-10)15(19)20/h4-7,14H,1-3,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli JM109 using HPPA as substrate after 10 mins |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
4-hydroxyphenylpyruvate dioxygenase
(Arabidopsis thaliana) | BDBM50264334
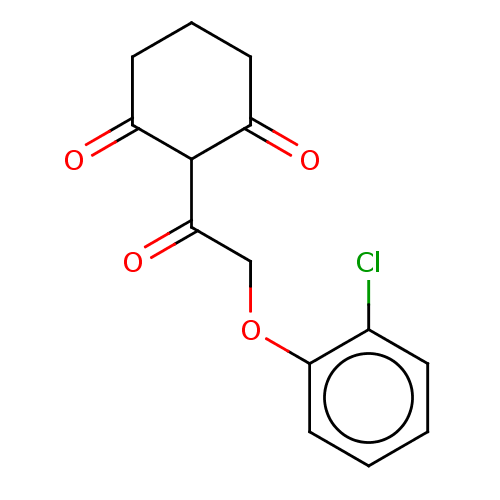
(CHEMBL4090319)Show InChI InChI=1S/C14H13ClO4/c15-9-4-1-2-7-13(9)19-8-12(18)14-10(16)5-3-6-11(14)17/h1-2,4,7,14H,3,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana HPPD expressed in Escherichia coli JM109 using HPPA as substrate after 10 mins |
J Med Chem 60: 4101-4125 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01395
BindingDB Entry DOI: 10.7270/Q2FN18ND |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224371

(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-(e...)Show SMILES CCSc1nc(Nc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19Cl2N5S/c1-2-29-21-26-19(25-16-10-6-9-15(22)11-16)17-12-24-28(20(17)27-21)13-18(23)14-7-4-3-5-8-14/h3-12,18H,2,13H2,1H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50230100

(4-fluoro-N-(5-(4-fluorobenzylthio)-1,3,4-thiadiazo...)Show InChI InChI=1S/C16H11F2N3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86859

(CAS_1327207 | N-(5-(4-bromobenzylthio)-1,3,4-thiad...)Show InChI InChI=1S/C16H11BrClN3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224388
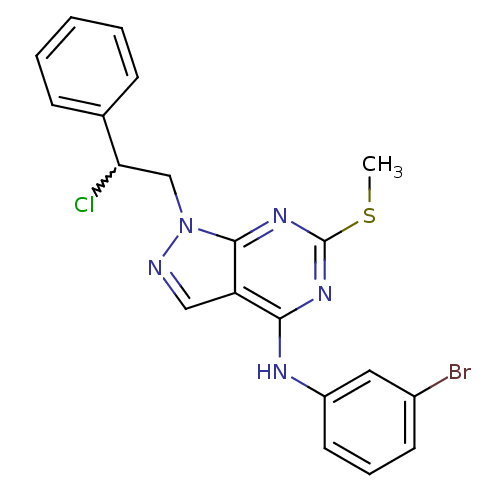
(CHEMBL238561 | N-(3-bromophenyl)-1-(2-chloro-2-phe...)Show SMILES CSc1nc(Nc2cccc(Br)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:18.19| Show InChI InChI=1S/C20H17BrClN5S/c1-28-20-25-18(24-15-9-5-8-14(21)10-15)16-11-23-27(19(16)26-20)12-17(22)13-6-3-2-4-7-13/h2-11,17H,12H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86863
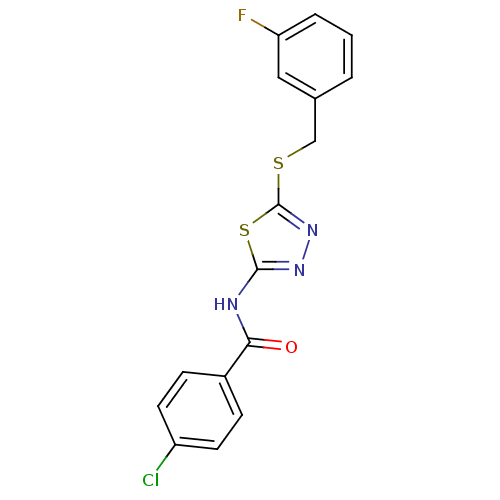
(CAS_4390766 | N-(5-(3-fluorobenzylthio)-1,3,4-thia...)Show InChI InChI=1S/C16H11ClFN3OS2/c17-12-6-4-11(5-7-12)14(22)19-15-20-21-16(24-15)23-9-10-2-1-3-13(18)8-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86875

(CAS_685639 | N-(5-(benzylthio)-1,3,4-thiadiazol-2-...)Show InChI InChI=1S/C16H12FN3OS2/c17-13-8-6-12(7-9-13)14(21)18-15-19-20-16(23-15)22-10-11-4-2-1-3-5-11/h1-9H,10H2,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86874

(CAS_44454498 | N-(5-(3-chlorobenzylthio)-1,3,4-thi...)Show InChI InChI=1S/C16H11Cl2N3OS2/c17-12-6-4-11(5-7-12)14(22)19-15-20-21-16(24-15)23-9-10-2-1-3-13(18)8-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224377
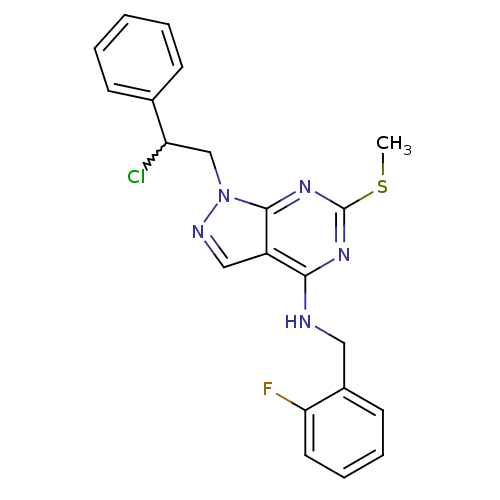
(1-(2-chloro-2-phenylethyl)-N-(2-fluorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2ccccc2F)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19ClFN5S/c1-29-21-26-19(24-11-15-9-5-6-10-18(15)23)16-12-25-28(20(16)27-21)13-17(22)14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86857

(CAS_44454939 | N-(5-(4-nitrobenzylthio)-1,3,4-thia...)Show SMILES [O-][N+](=O)c1ccc(CSc2nnc(NC(=O)c3ccccc3Cl)s2)cc1 Show InChI InChI=1S/C16H11ClN4O3S2/c17-13-4-2-1-3-12(13)14(22)18-15-19-20-16(26-15)25-9-10-5-7-11(8-6-10)21(23)24/h1-8H,9H2,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86868
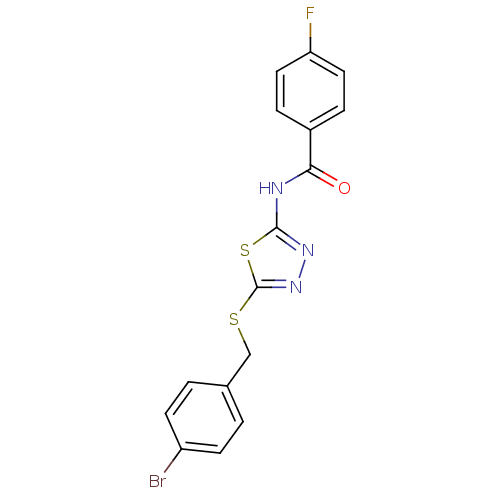
(CAS_3643794 | N-(5-(4-bromobenzylthio)-1,3,4-thiad...)Show InChI InChI=1S/C16H11BrFN3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224387
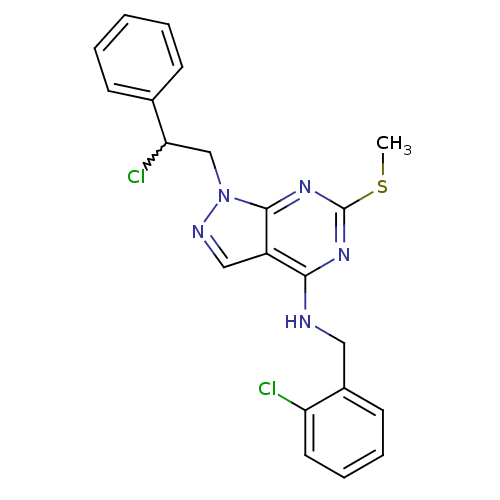
(1-(2-chloro-2-phenylethyl)-N-(2-chlorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2ccccc2Cl)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19Cl2N5S/c1-29-21-26-19(24-11-15-9-5-6-10-17(15)22)16-12-25-28(20(16)27-21)13-18(23)14-7-3-2-4-8-14/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86856

(CAS_44454904 | N-(5-(4-nitrobenzylthio)-1,3,4-thia...)Show SMILES [O-][N+](=O)c1ccc(CSc2nnc(NC(=O)c3ccc(Cl)cc3)s2)cc1 Show InChI InChI=1S/C16H11ClN4O3S2/c17-12-5-3-11(4-6-12)14(22)18-15-19-20-16(26-15)25-9-10-1-7-13(8-2-10)21(23)24/h1-8H,9H2,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224372
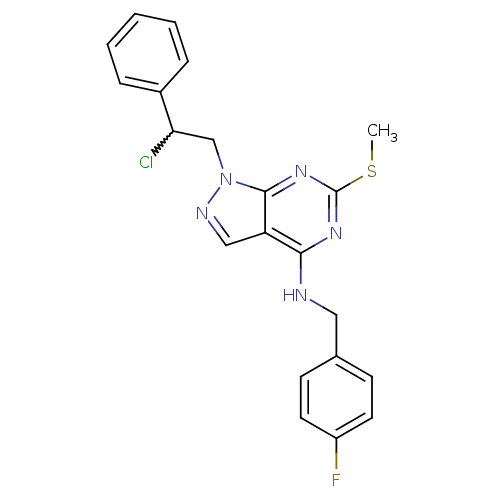
(1-(2-chloro-2-phenylethyl)-N-(4-fluorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2ccc(F)cc2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19ClFN5S/c1-29-21-26-19(24-11-14-7-9-16(23)10-8-14)17-12-25-28(20(17)27-21)13-18(22)15-5-3-2-4-6-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86860

(CAS_3320545 | N-(5-(4-fluorobenzylthio)-1,3,4-thia...)Show InChI InChI=1S/C16H11ClFN3OS2/c17-12-5-3-11(4-6-12)14(22)19-15-20-21-16(24-15)23-9-10-1-7-13(18)8-2-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224383

(1-(2-chloro-2-(4-chlorophenyl)ethyl)-6-(methylthio...)Show SMILES CSc1nc(NCCc2ccccc2)c2cnn(CC(Cl)c3ccc(Cl)cc3)c2n1 |w:19.20| Show InChI InChI=1S/C22H21Cl2N5S/c1-30-22-27-20(25-12-11-15-5-3-2-4-6-15)18-13-26-29(21(18)28-22)14-19(24)16-7-9-17(23)10-8-16/h2-10,13,19H,11-12,14H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224374
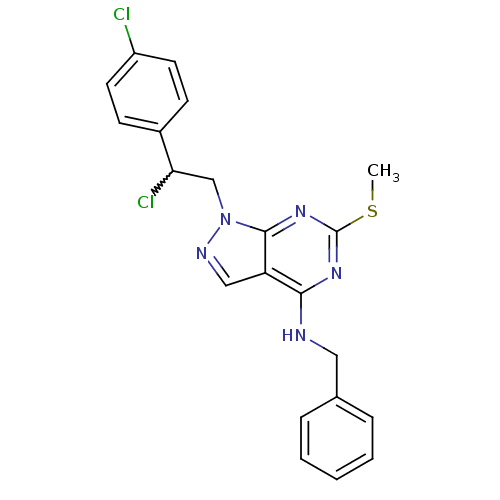
(CHEMBL241749 | N-benzyl-1-(2-chloro-2-(4-chlorophe...)Show SMILES CSc1nc(NCc2ccccc2)c2cnn(CC(Cl)c3ccc(Cl)cc3)c2n1 |w:18.19| Show InChI InChI=1S/C21H19Cl2N5S/c1-29-21-26-19(24-11-14-5-3-2-4-6-14)17-12-25-28(20(17)27-21)13-18(23)15-7-9-16(22)10-8-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224369
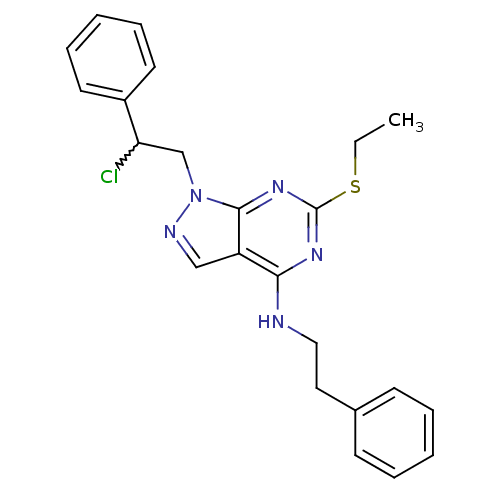
(1-(2-chloro-2-phenylethyl)-6-(ethylthio)-N-pheneth...)Show SMILES CCSc1nc(NCCc2ccccc2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C23H24ClN5S/c1-2-30-23-27-21(25-14-13-17-9-5-3-6-10-17)19-15-26-29(22(19)28-23)16-20(24)18-11-7-4-8-12-18/h3-12,15,20H,2,13-14,16H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224382
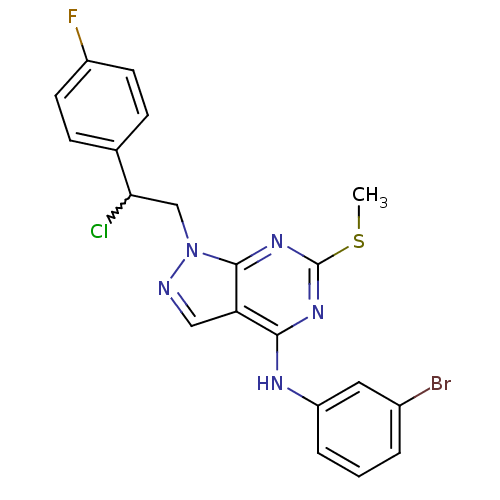
(CHEMBL399627 | N-(3-bromophenyl)-1-(2-chloro-2-(4-...)Show SMILES CSc1nc(Nc2cccc(Br)c2)c2cnn(CC(Cl)c3ccc(F)cc3)c2n1 |w:18.19| Show InChI InChI=1S/C20H16BrClFN5S/c1-29-20-26-18(25-15-4-2-3-13(21)9-15)16-10-24-28(19(16)27-20)11-17(22)12-5-7-14(23)8-6-12/h2-10,17H,11H2,1H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224376
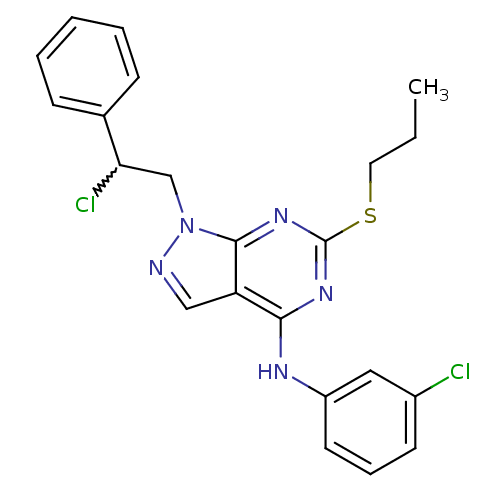
(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-(p...)Show SMILES CCCSc1nc(Nc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21Cl2N5S/c1-2-11-30-22-27-20(26-17-10-6-9-16(23)12-17)18-13-25-29(21(18)28-22)14-19(24)15-7-4-3-5-8-15/h3-10,12-13,19H,2,11,14H2,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86856

(CAS_44454904 | N-(5-(4-nitrobenzylthio)-1,3,4-thia...)Show SMILES [O-][N+](=O)c1ccc(CSc2nnc(NC(=O)c3ccc(Cl)cc3)s2)cc1 Show InChI InChI=1S/C16H11ClN4O3S2/c17-12-5-3-11(4-6-12)14(22)18-15-19-20-16(26-15)25-9-10-1-7-13(8-2-10)21(23)24/h1-8H,9H2,(H,18,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86867
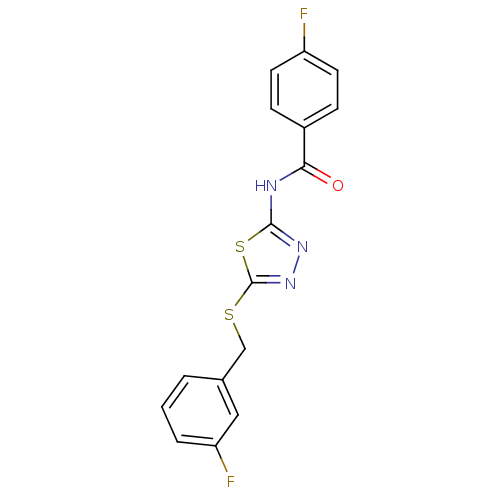
(CAS_4573026 | N-(5-(3-fluorobenzylthio)-1,3,4-thia...)Show InChI InChI=1S/C16H11F2N3OS2/c17-12-6-4-11(5-7-12)14(22)19-15-20-21-16(24-15)23-9-10-2-1-3-13(18)8-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86866

(CAS_44454722 | N-(5-(3-chlorobenzylthio)-1,3,4-thi...)Show InChI InChI=1S/C16H11Cl2N3OS2/c17-11-5-3-4-10(8-11)9-23-16-21-20-15(24-16)19-14(22)12-6-1-2-7-13(12)18/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86870

(CAS_44454776 | N-(5-(4-nitrobenzylthio)-1,3,4-thia...)Show SMILES [O-][N+](=O)c1ccc(CSc2nnc(NC(=O)c3ccc(F)cc3)s2)cc1 Show InChI InChI=1S/C16H11FN4O3S2/c17-12-5-3-11(4-6-12)14(22)18-15-19-20-16(26-15)25-9-10-1-7-13(8-2-10)21(23)24/h1-8H,9H2,(H,18,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86864

(CAS_44454803 | N-(5-(4-methoxybenzylthio)-1,3,4-th...)Show InChI InChI=1S/C17H14ClN3O2S2/c1-23-14-8-2-11(3-9-14)10-24-17-21-20-16(25-17)19-15(22)12-4-6-13(18)7-5-12/h2-9H,10H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86874

(CAS_44454498 | N-(5-(3-chlorobenzylthio)-1,3,4-thi...)Show InChI InChI=1S/C16H11Cl2N3OS2/c17-12-6-4-11(5-7-12)14(22)19-15-20-21-16(24-15)23-9-10-2-1-3-13(18)8-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86872

(CAS_42805643 | N-(5-(4-methoxybenzylthio)-1,3,4-th...)Show InChI InChI=1S/C17H14FN3O2S2/c1-23-14-8-2-11(3-9-14)10-24-17-21-20-16(25-17)19-15(22)12-4-6-13(18)7-5-12/h2-9H,10H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86864

(CAS_44454803 | N-(5-(4-methoxybenzylthio)-1,3,4-th...)Show InChI InChI=1S/C17H14ClN3O2S2/c1-23-14-8-2-11(3-9-14)10-24-17-21-20-16(25-17)19-15(22)12-4-6-13(18)7-5-12/h2-9H,10H2,1H3,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224380
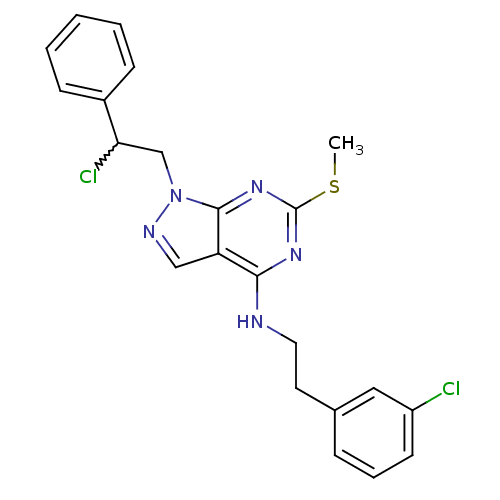
(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenethyl)-6...)Show SMILES CSc1nc(NCCc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21Cl2N5S/c1-30-22-27-20(25-11-10-15-6-5-9-17(23)12-15)18-13-26-29(21(18)28-22)14-19(24)16-7-3-2-4-8-16/h2-9,12-13,19H,10-11,14H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86860

(CAS_3320545 | N-(5-(4-fluorobenzylthio)-1,3,4-thia...)Show InChI InChI=1S/C16H11ClFN3OS2/c17-12-5-3-11(4-6-12)14(22)19-15-20-21-16(24-15)23-9-10-1-7-13(18)8-2-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86870

(CAS_44454776 | N-(5-(4-nitrobenzylthio)-1,3,4-thia...)Show SMILES [O-][N+](=O)c1ccc(CSc2nnc(NC(=O)c3ccc(F)cc3)s2)cc1 Show InChI InChI=1S/C16H11FN4O3S2/c17-12-5-3-11(4-6-12)14(22)18-15-19-20-16(26-15)25-9-10-1-7-13(8-2-10)21(23)24/h1-8H,9H2,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86859

(CAS_1327207 | N-(5-(4-bromobenzylthio)-1,3,4-thiad...)Show InChI InChI=1S/C16H11BrClN3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86863

(CAS_4390766 | N-(5-(3-fluorobenzylthio)-1,3,4-thia...)Show InChI InChI=1S/C16H11ClFN3OS2/c17-12-6-4-11(5-7-12)14(22)19-15-20-21-16(24-15)23-9-10-2-1-3-13(18)8-10/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86857

(CAS_44454939 | N-(5-(4-nitrobenzylthio)-1,3,4-thia...)Show SMILES [O-][N+](=O)c1ccc(CSc2nnc(NC(=O)c3ccccc3Cl)s2)cc1 Show InChI InChI=1S/C16H11ClN4O3S2/c17-13-4-2-1-3-12(13)14(22)18-15-19-20-16(26-15)25-9-10-5-7-11(8-6-10)21(23)24/h1-8H,9H2,(H,18,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM86868

(CAS_3643794 | N-(5-(4-bromobenzylthio)-1,3,4-thiad...)Show InChI InChI=1S/C16H11BrFN3OS2/c17-12-5-1-10(2-6-12)9-23-16-21-20-15(24-16)19-14(22)11-3-7-13(18)8-4-11/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM86873
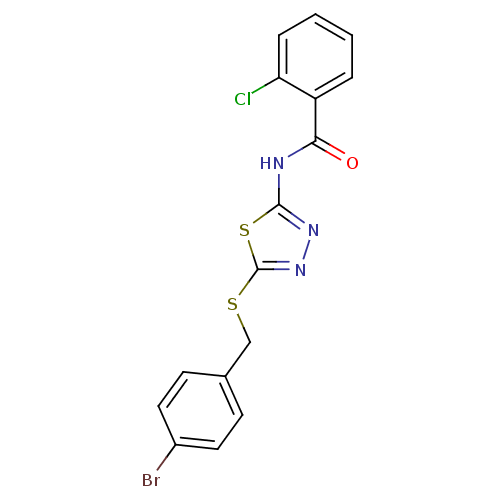
(CAS_3448811 | N-(5-(4-bromobenzylthio)-1,3,4-thiad...)Show InChI InChI=1S/C16H11BrClN3OS2/c17-11-7-5-10(6-8-11)9-23-16-21-20-15(24-16)19-14(22)12-3-1-2-4-13(12)18/h1-8H,9H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 1207-11 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.112
BindingDB Entry DOI: 10.7270/Q23R0RF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50224373

(1-(2-chloro-2-phenylethyl)-N-(3-fluorophenethyl)-6...)Show SMILES CSc1nc(NCCc2cccc(F)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21ClFN5S/c1-30-22-27-20(25-11-10-15-6-5-9-17(24)12-15)18-13-26-29(21(18)28-22)14-19(23)16-7-3-2-4-8-16/h2-9,12-13,19H,10-11,14H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Abl |
Eur J Med Chem 44: 3712-7 (2009)
Article DOI: 10.1016/j.ejmech.2009.03.039
BindingDB Entry DOI: 10.7270/Q2SF2W6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data