

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249294 ((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249114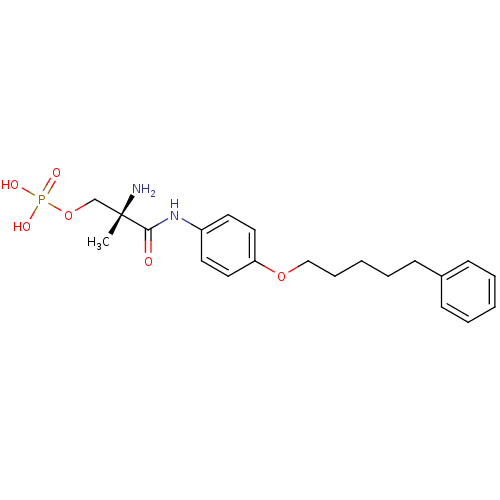 ((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315562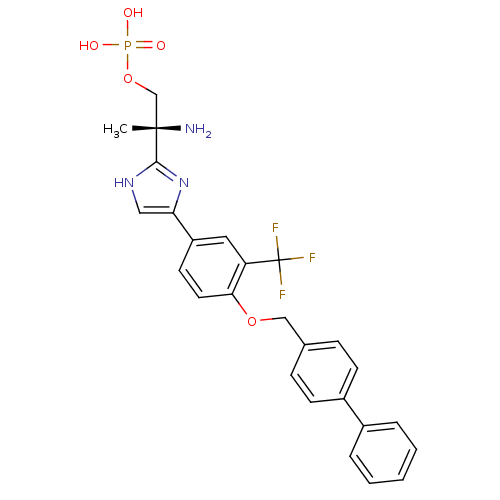 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315559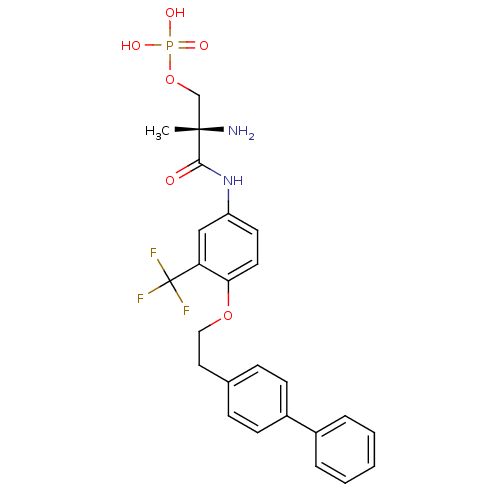 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50249114 ((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249259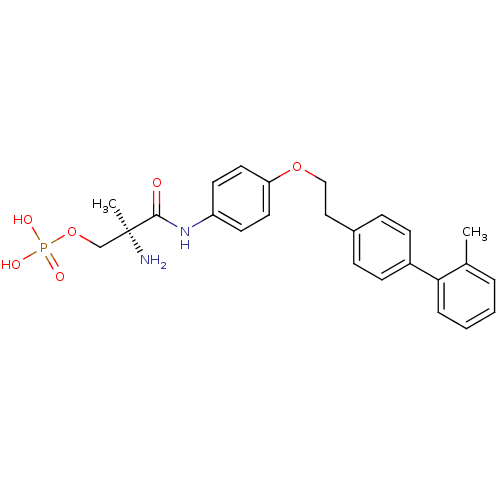 ((S)-2-amino-2-methyl-3-(4-(2-(2'-methylbiphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315556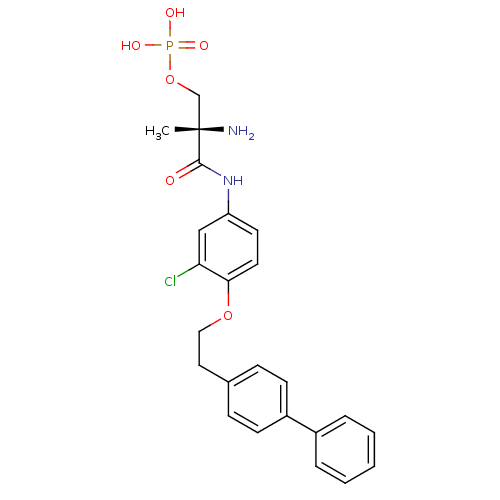 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249260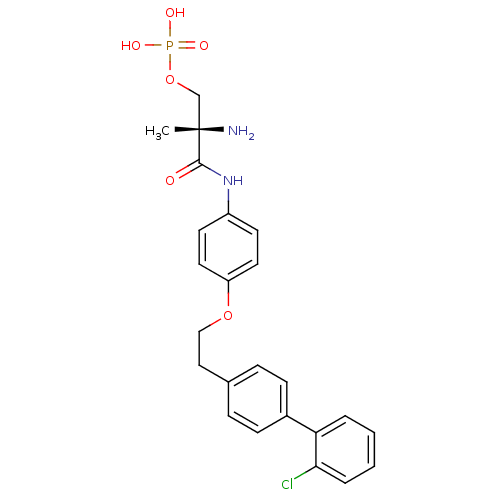 ((S)-2-amino-3-(4-(2-(2'-chlorobiphenyl-4-yl)ethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249263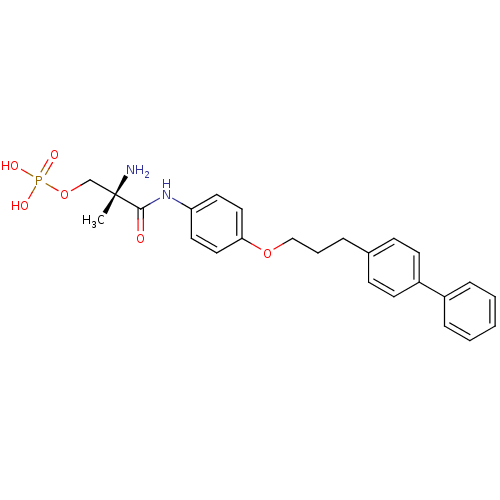 ((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propoxy)phenyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249293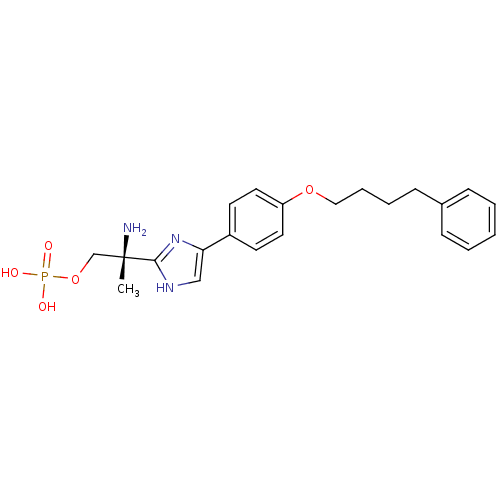 ((R)-2-amino-2-(4-(4-(4-phenylbutoxy)phenyl)-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450546 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[6-[1-(2-hydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450548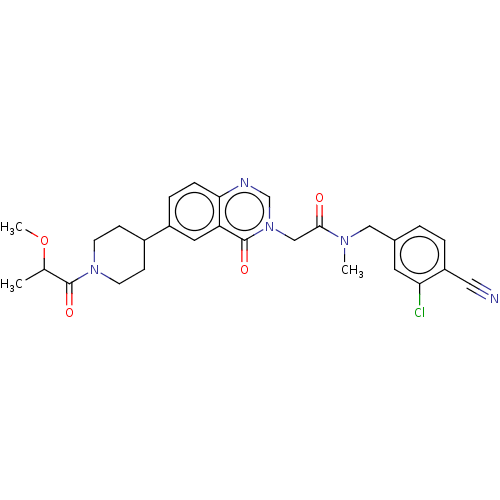 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450606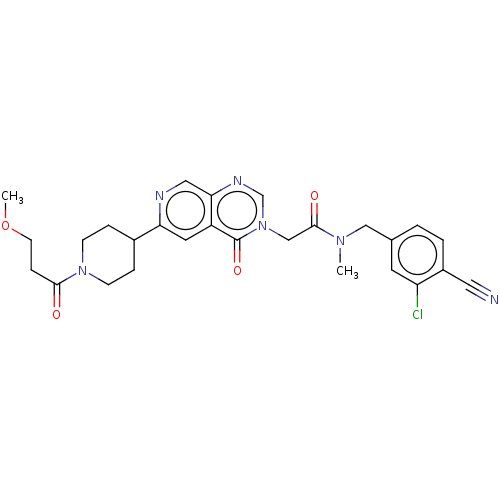 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[6-[1-(3-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450605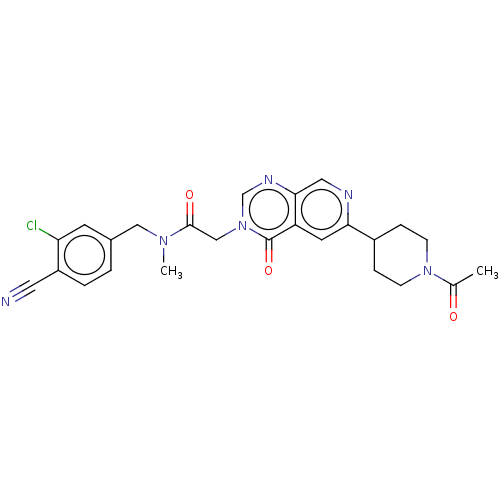 (2-[6-(1-Acetylpiperidin-4-yl)-4-oxopyrido[3,4-d]py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450604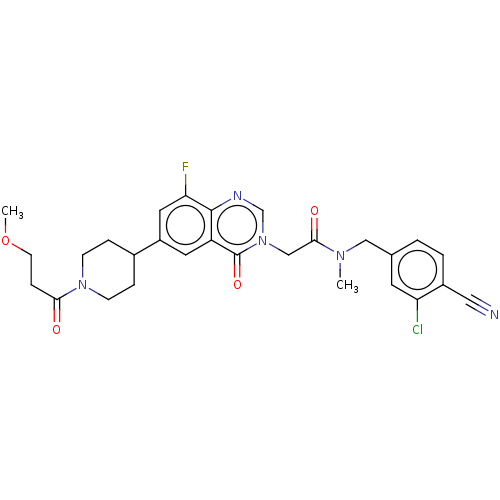 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[8-fluoro-6-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450603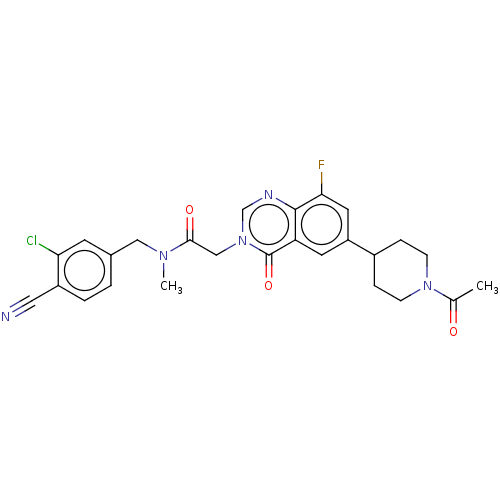 (2-[6-(1-Acetylpiperidin-4-yl)-8-fluoro-4-oxoquinaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450579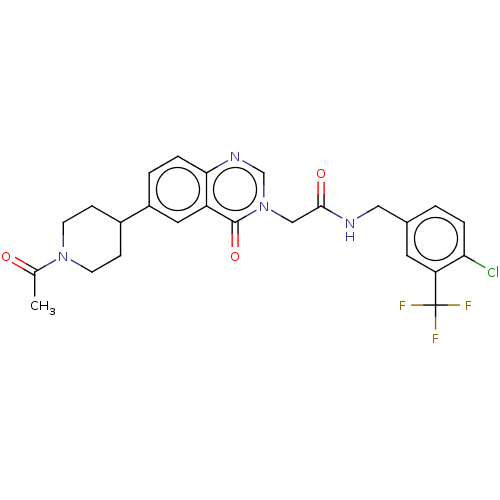 (2-[6-(1-tert-Butoxycarbonyl-4- piperidyl)-4-oxoqui...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450575 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450549 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450551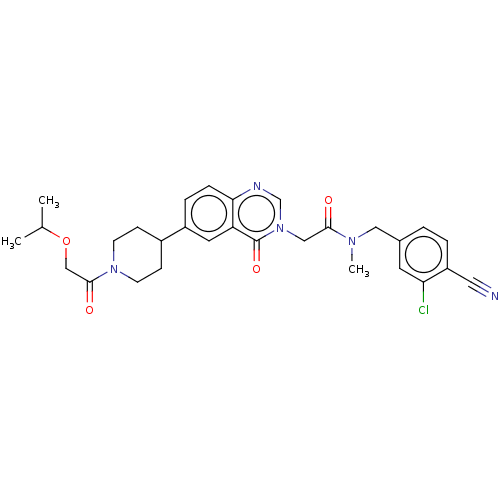 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450552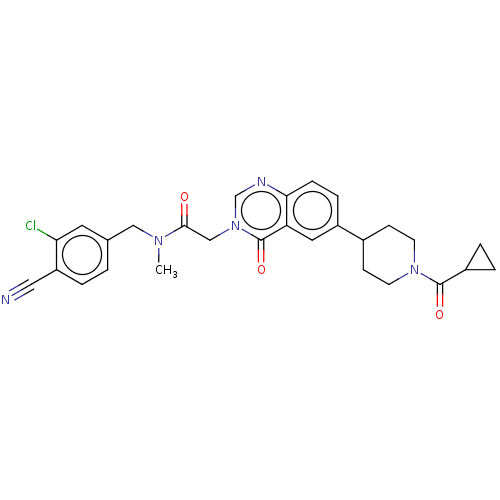 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450562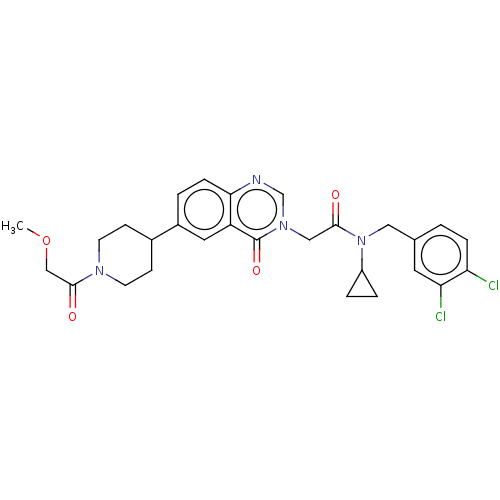 (N-Cyclopropyl-N-[(3,4-dichlorophenyl)methyl]-2-[6-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249266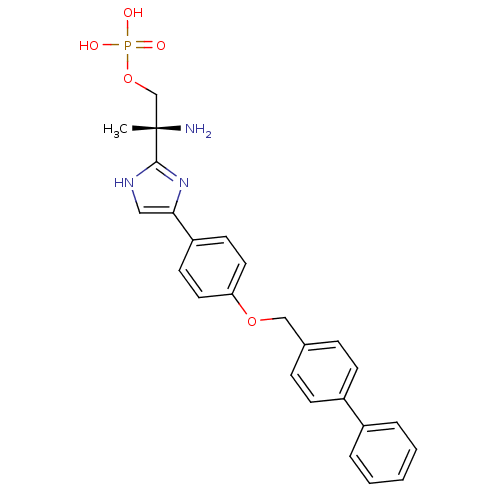 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249266 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249240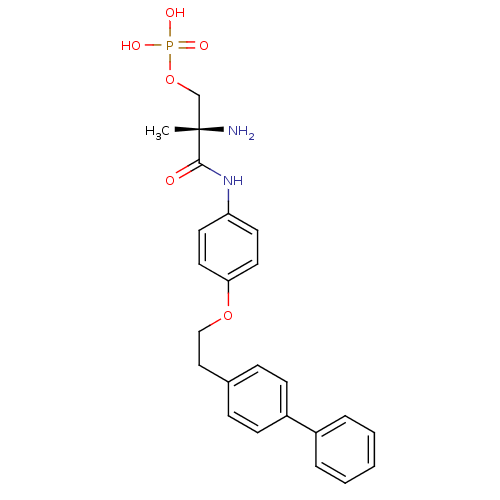 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249240 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249153 ((S)-2-amino-3-(4-(4-cyclohexylbutoxy)phenylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50249294 ((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315557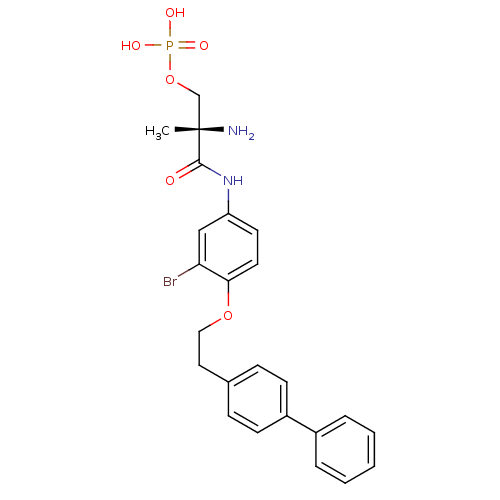 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-bromo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450553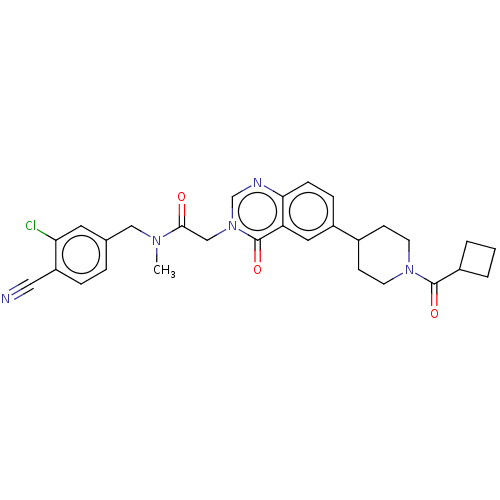 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450557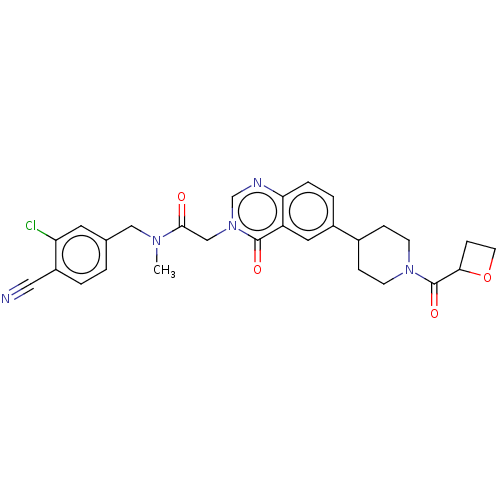 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450570 (N-[(3-Chloro-4-cyanophenyl)methyl]-2-[6-[1-(2-cyan...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450574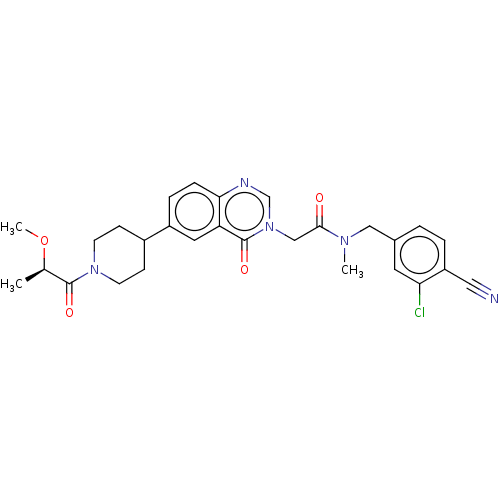 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450590 (N-[(3,4- Dichlorophenyl)methyl]-N- methyl-2-(4-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450489 (US10676446, Example 84) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36463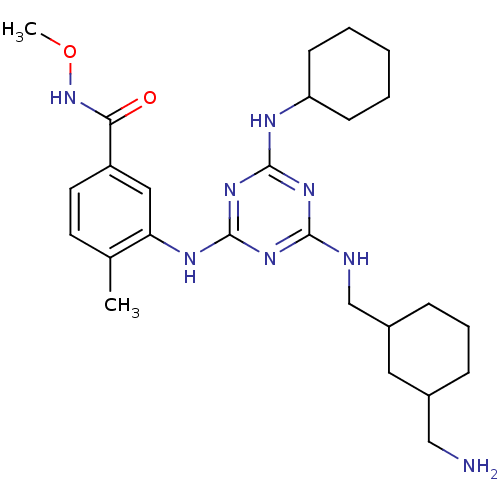 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36462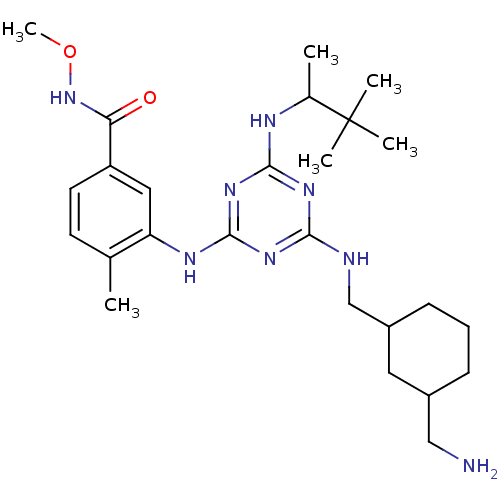 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249113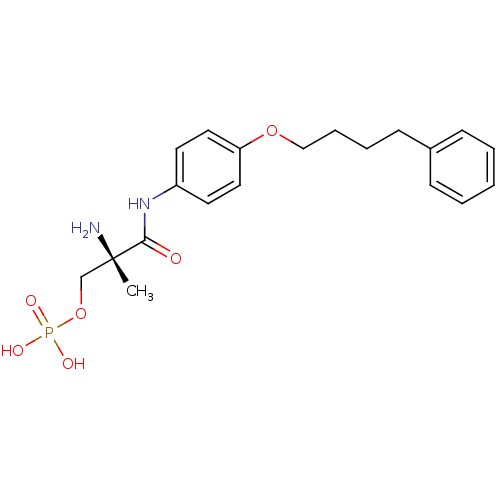 ((S)-2-amino-2-methyl-3-oxo-3-(4-(4-phenylbutoxy)ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249154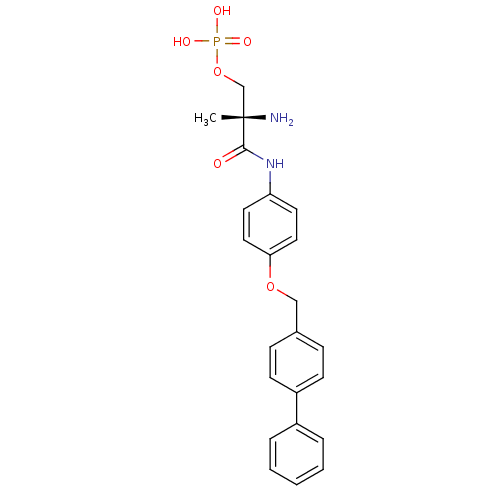 ((S)-2-amino-3-(4-(biphenyl-4-ylmethoxy)phenylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50249114 ((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450554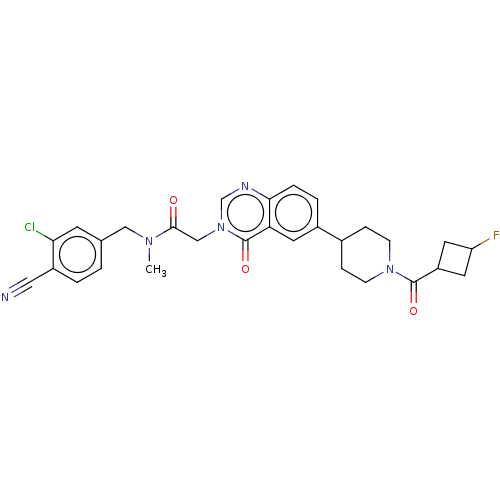 (N-[(3-Chloro-4- cyanophenyl)methyl]-N- methyl-2-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450587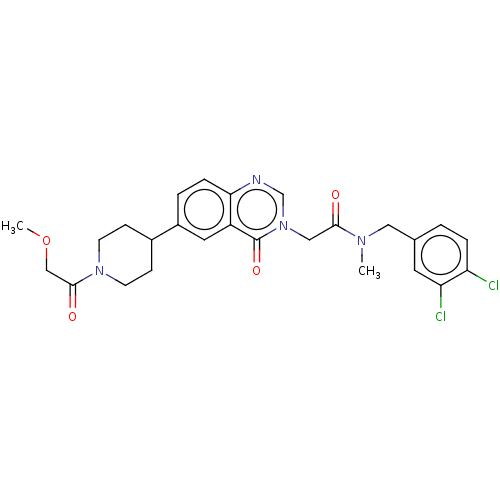 (N-[(3,4- Dichlorophenyl)methyl]-N- methyl-2-(4-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450460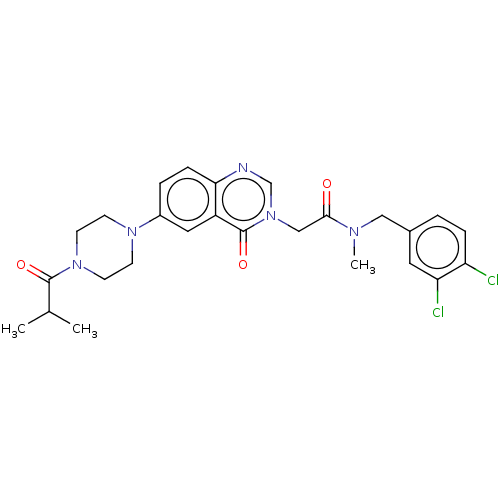 (9H-Fluoren-9-ylmethyl 4-[3-[2- [(3,4-dichloropheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450473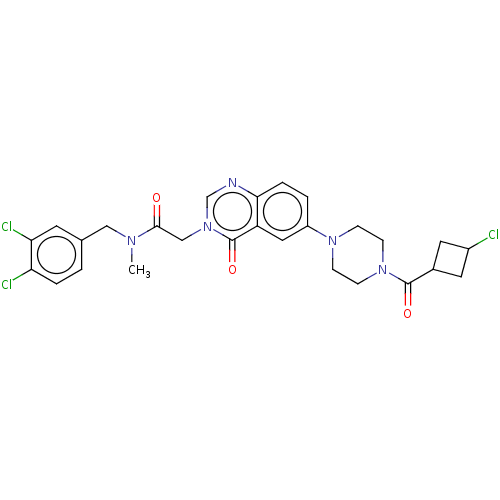 (9H-Fluoren-9-ylmethyl 4-[3-[2-[(3,4- dichloropheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315555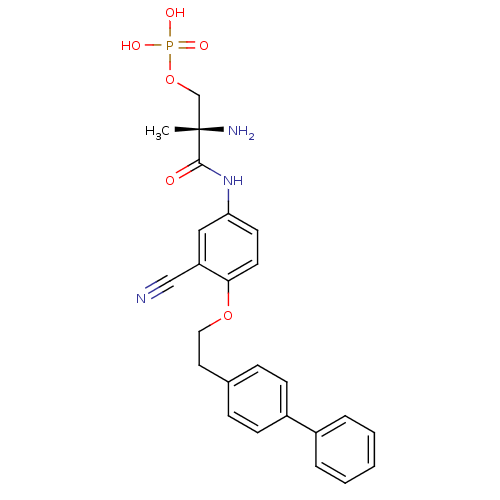 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-cyano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50315559 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50249294 ((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50249114 ((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor | Bioorg Med Chem Lett 19: 2315-9 (2009) Article DOI: 10.1016/j.bmcl.2009.02.073 BindingDB Entry DOI: 10.7270/Q2XD11KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450504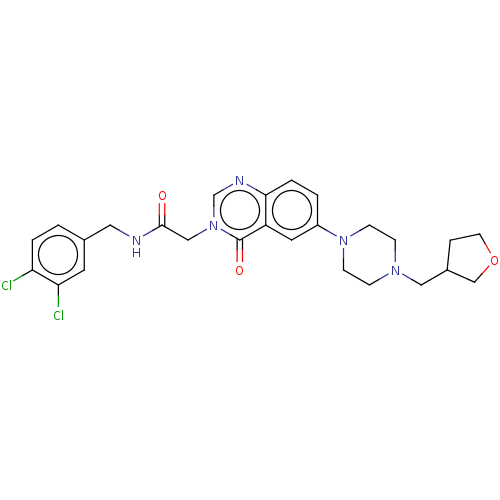 (9H-Fluoren-9- ylmethyl 4-[3- [2-[(3,4- dichlorophe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM450583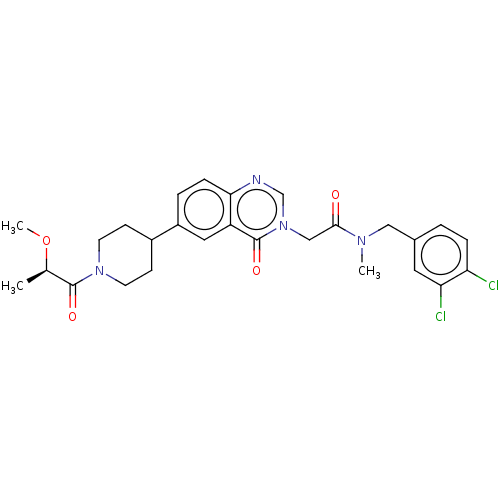 (N-[(3,4- Dichlorophenyl)methyl]-N- methyl-2-(4-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121... | US Patent US10676446 (2020) BindingDB Entry DOI: 10.7270/Q2445QJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 818 total ) | Next | Last >> |