

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139210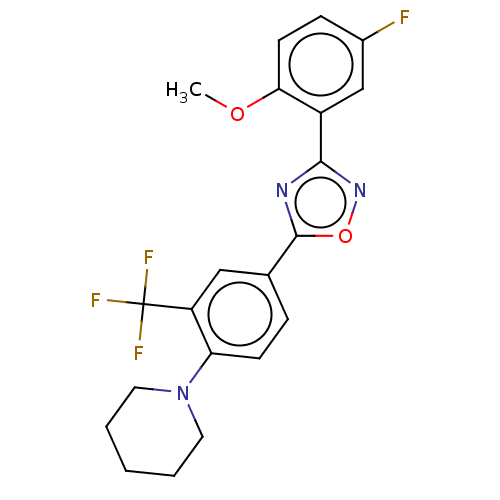 (US8889668, I60) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139202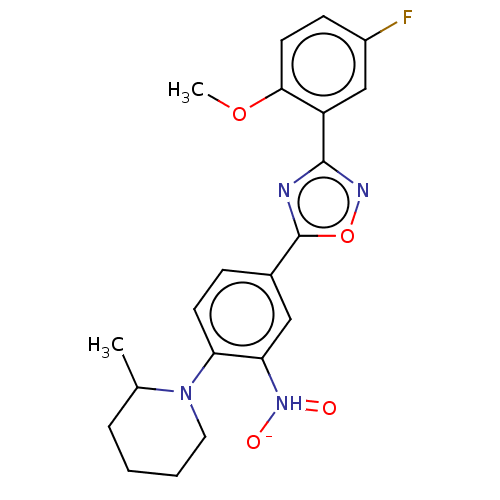 (US8889668, I32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139198 (US8889668, I26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139209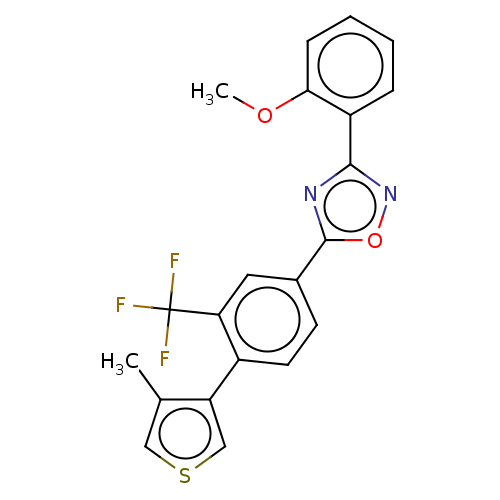 (US8889668, I57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131550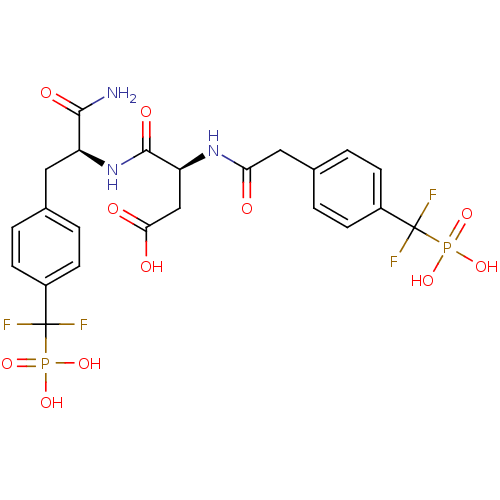 ((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B | J Med Chem 47: 4142-6 (2004) Article DOI: 10.1021/jm030629n BindingDB Entry DOI: 10.7270/Q2571BGC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139213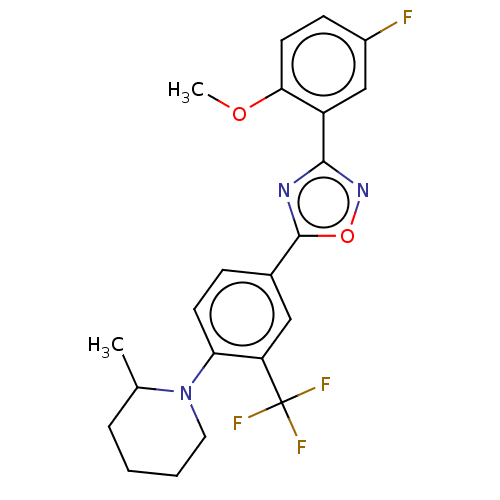 (US8889668, I63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139205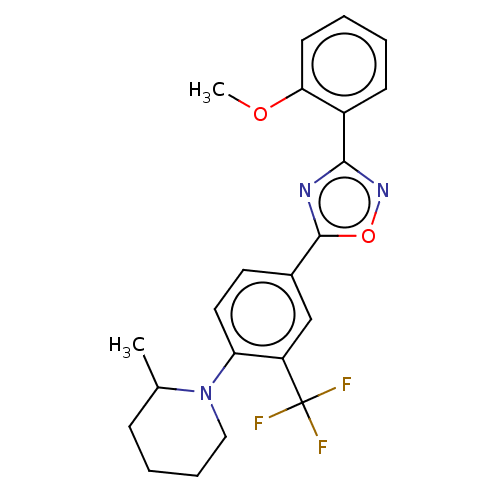 (US8889668, I35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139197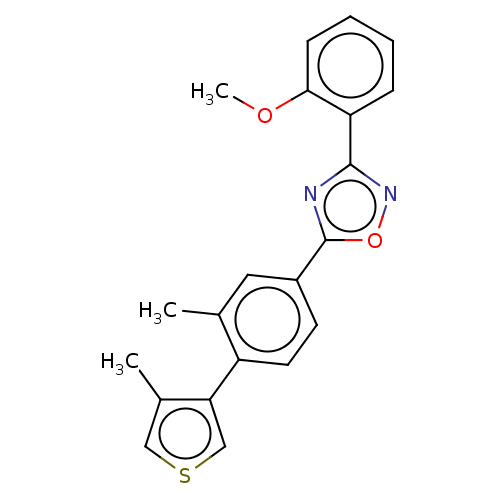 (US8889668, I25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139219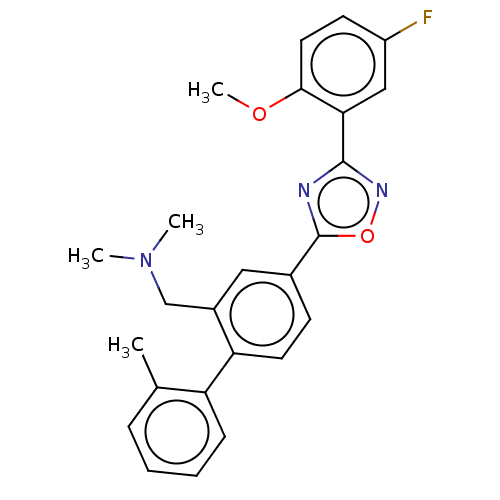 (US8889668, I78) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139215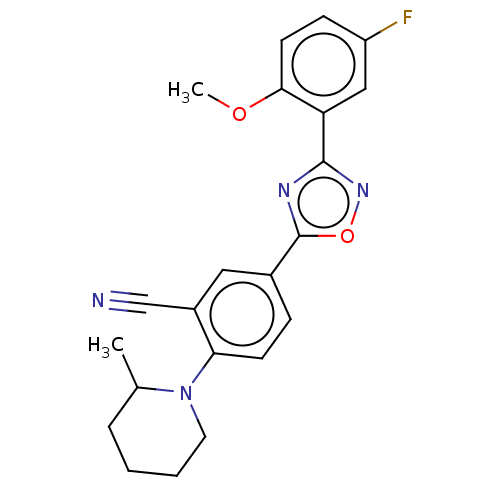 (US8889668, I71) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139207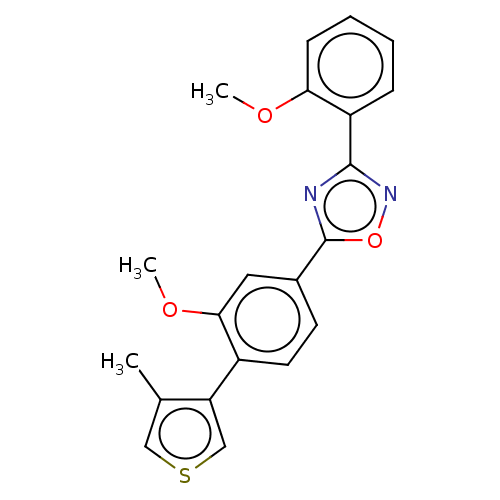 (US8889668, I48) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139214 (US8889668, I70) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139206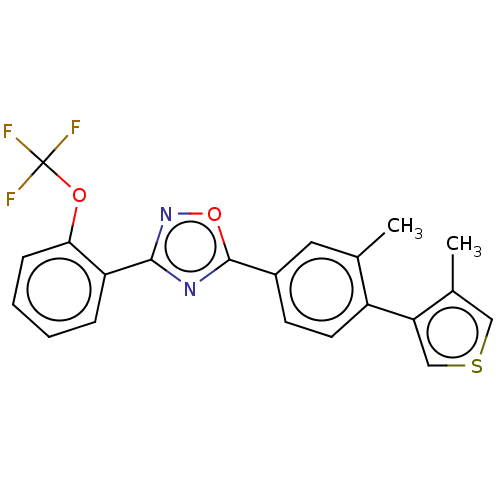 (US8889668, I44) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139204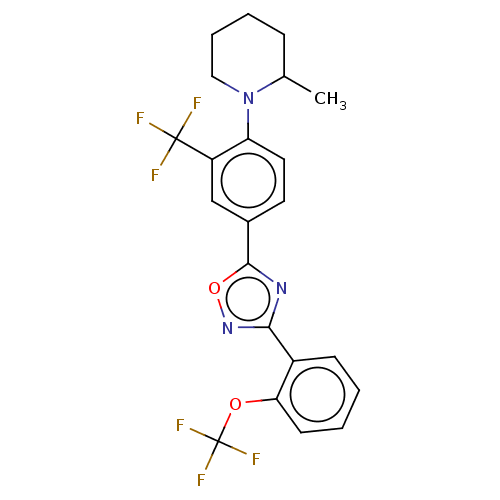 (US8889668, I34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139217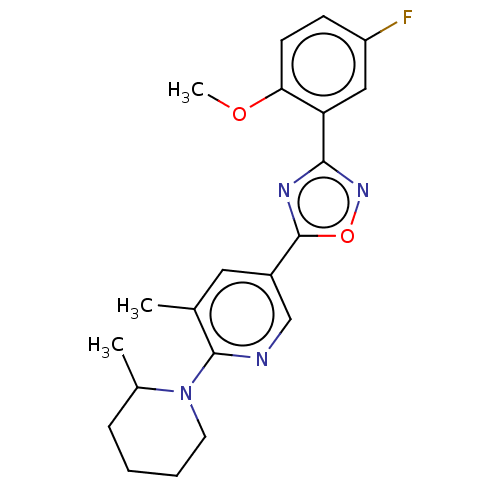 (US8889668, I74) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139193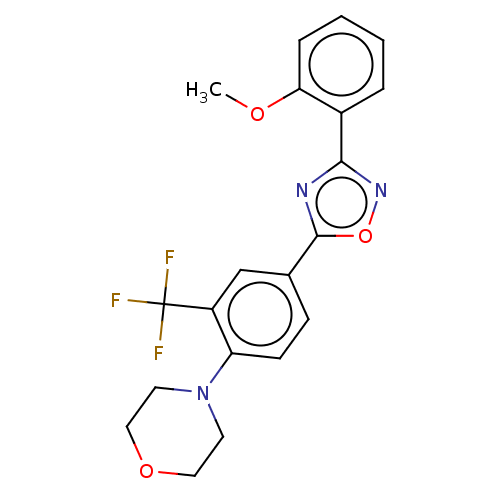 (US8889668, I18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139212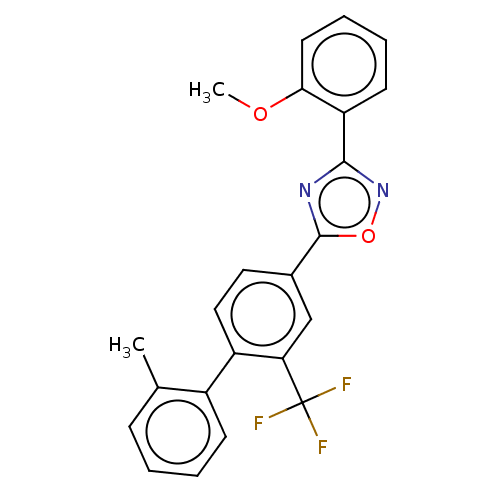 (US8889668, I62) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139208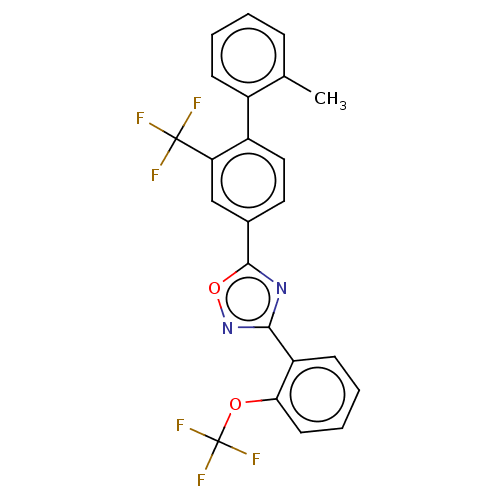 (US8889668, I55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139195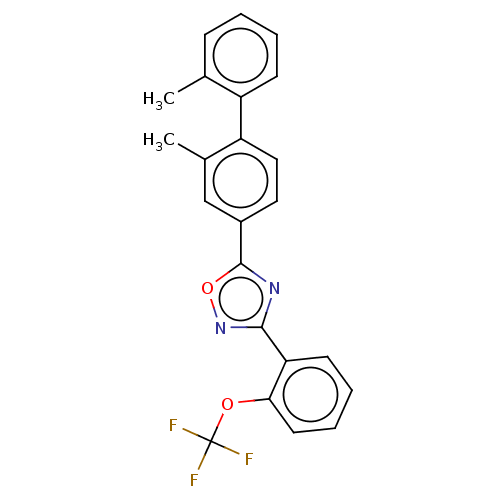 (US8889668, I21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139189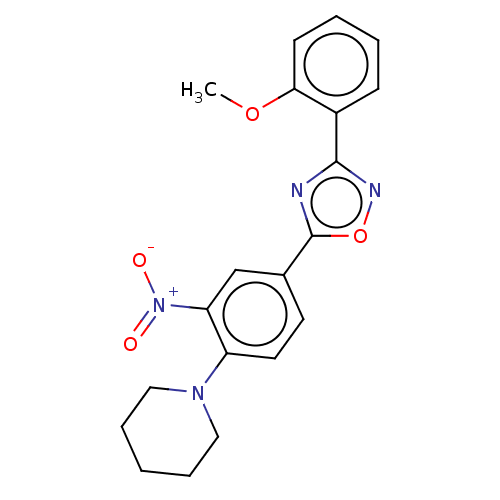 (US8889668, I1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139194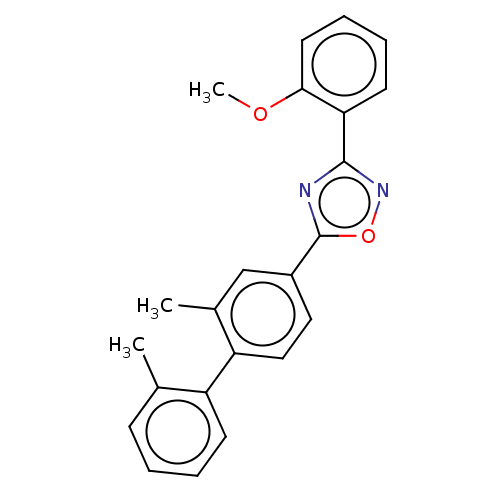 (US8889668, I20) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139196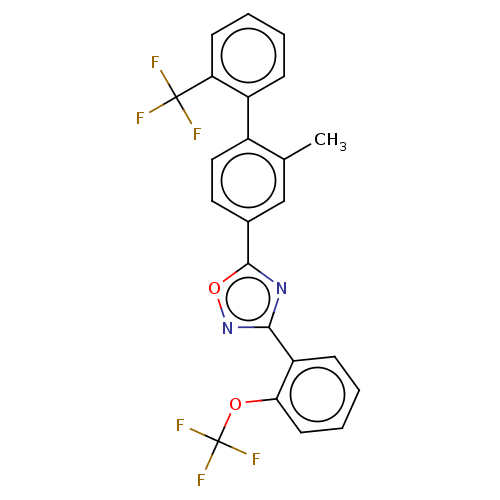 (US8889668, I23) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139190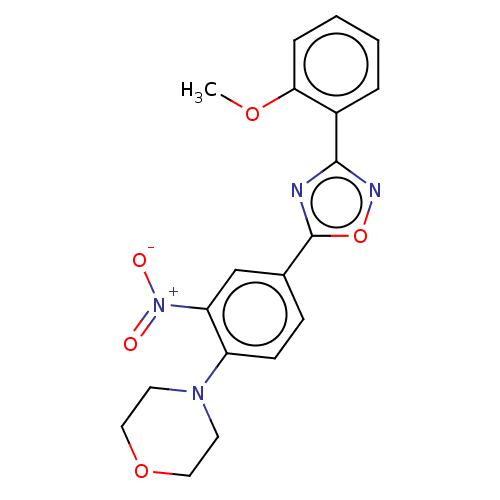 (US8889668, I2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 18 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50151008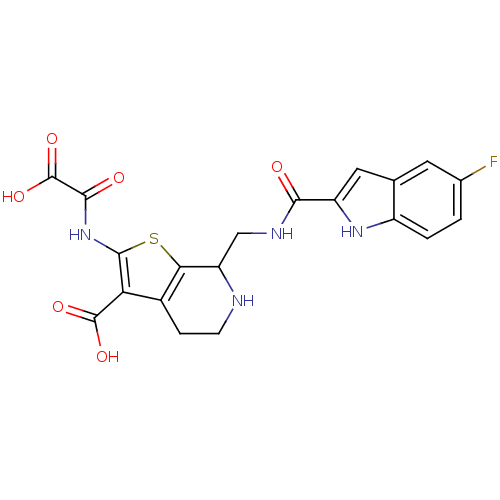 ((7S)-2-[(carboxycarbonyl)amino]-7-({[(5-fluoro-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B at pH 5.5 | J Med Chem 47: 4142-6 (2004) Article DOI: 10.1021/jm030629n BindingDB Entry DOI: 10.7270/Q2571BGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139199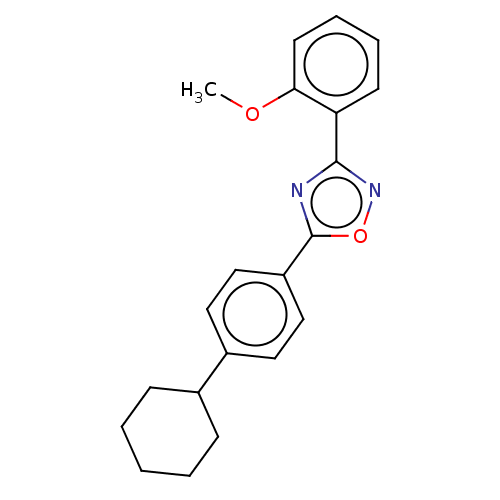 (US8889668, I27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139201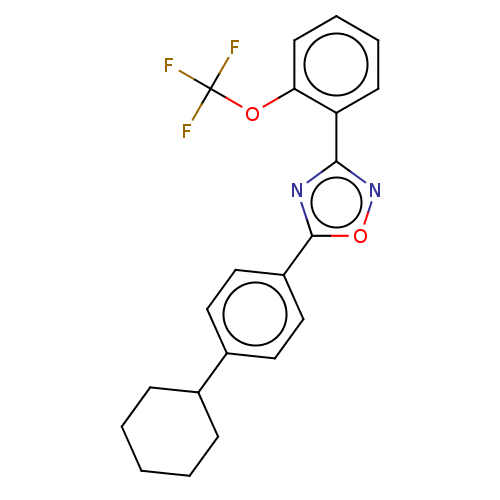 (US8889668, I29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139203 (US8889668, I33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139216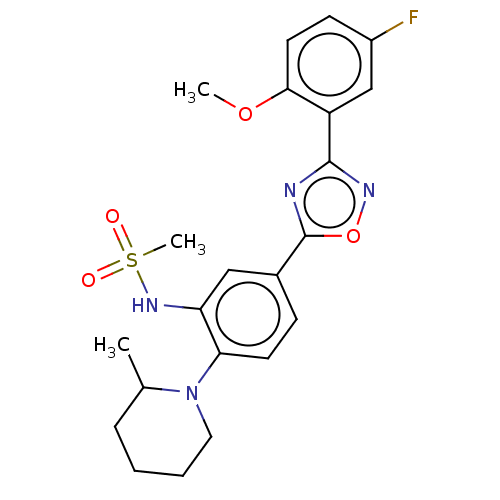 (US8889668, I72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139218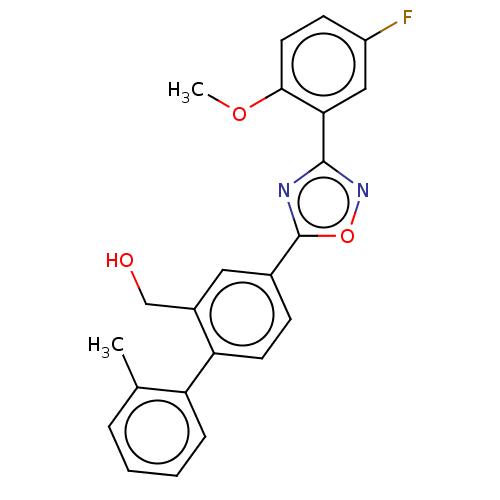 (US8889668, I77) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 53 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139211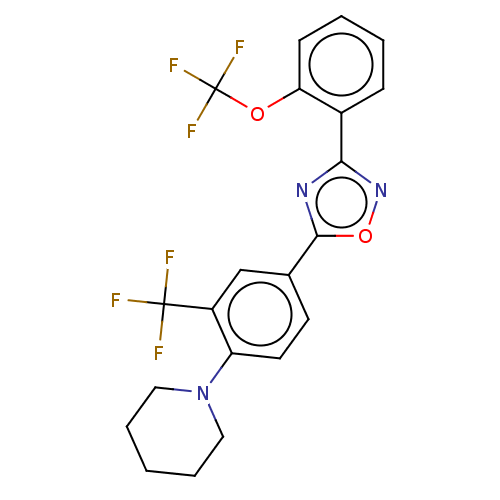 (US8889668, I61) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 54 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM15819 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B | J Med Chem 47: 4142-6 (2004) Article DOI: 10.1021/jm030629n BindingDB Entry DOI: 10.7270/Q2571BGC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139200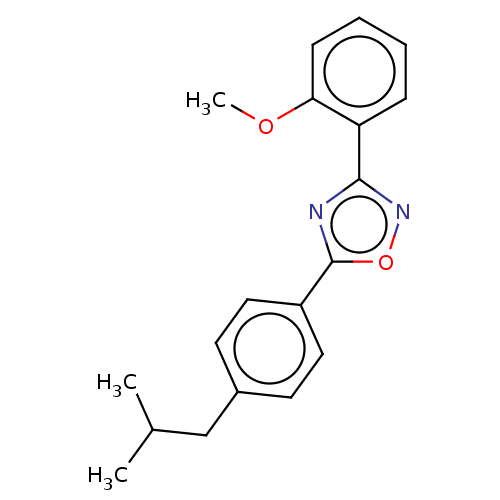 (US8889668, I28) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 91 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139191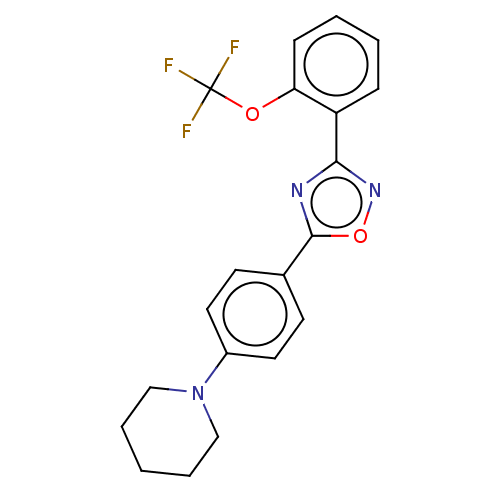 (US8889668, I3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 652 | -32.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM139192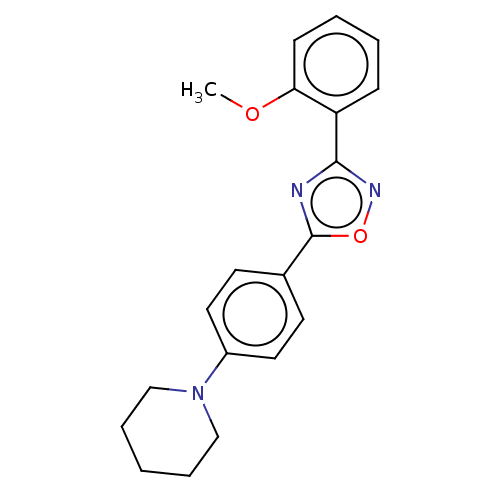 (US8889668, I4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.07E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells wer... | US Patent US8889668 (2014) BindingDB Entry DOI: 10.7270/Q2V123G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128379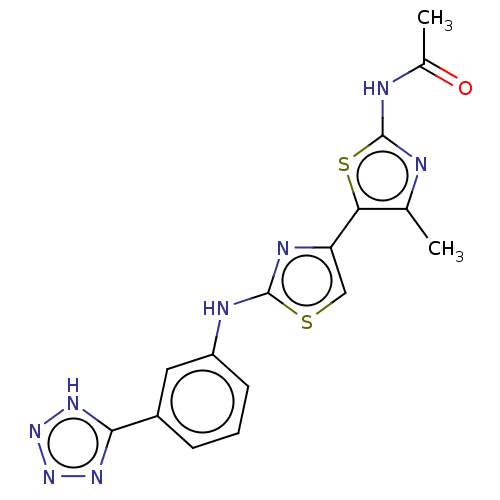 (US8802861, 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50402265 (CHEMBL2164280 | US8802861, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128377 (US8802861, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128383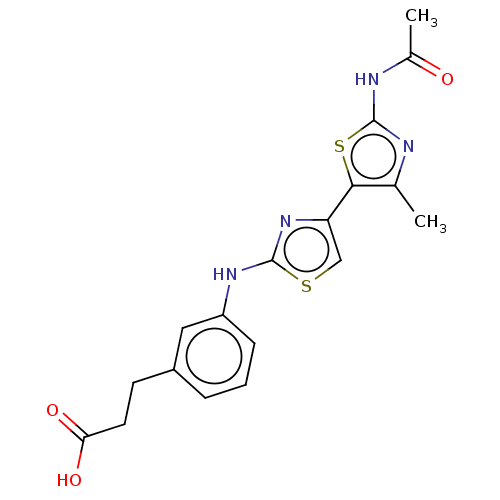 (US8802861, 136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128382 (US8802861, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128376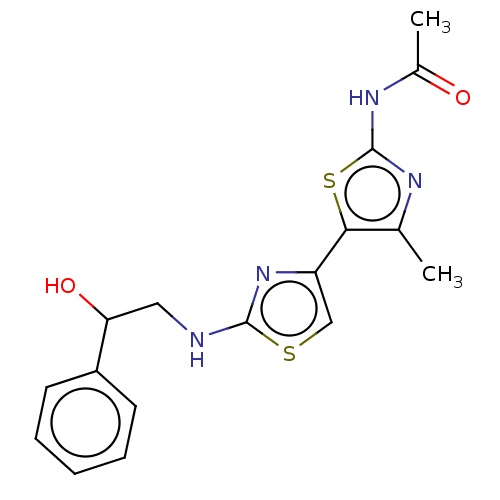 (US8802861, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128370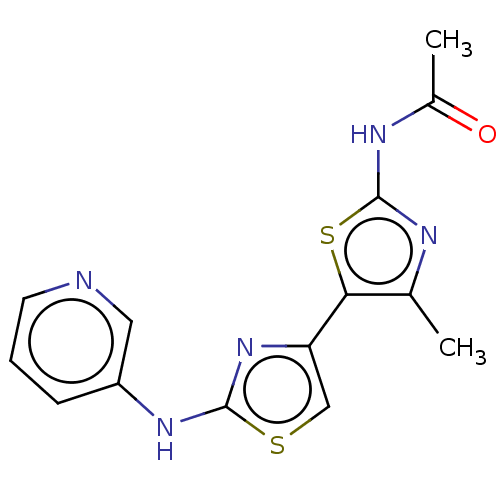 (US8802861, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50158551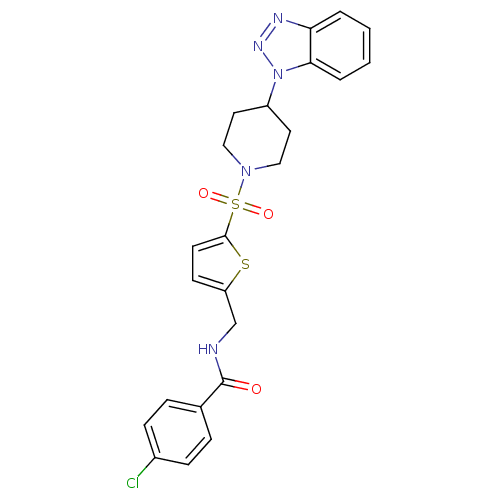 (CHEMBL383955 | N-((5-(4-(1H-benzo[d][1,2,3]triazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human JNK3 | J Med Chem 47: 6921-34 (2004) Article DOI: 10.1021/jm031112e BindingDB Entry DOI: 10.7270/Q2XG9QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128381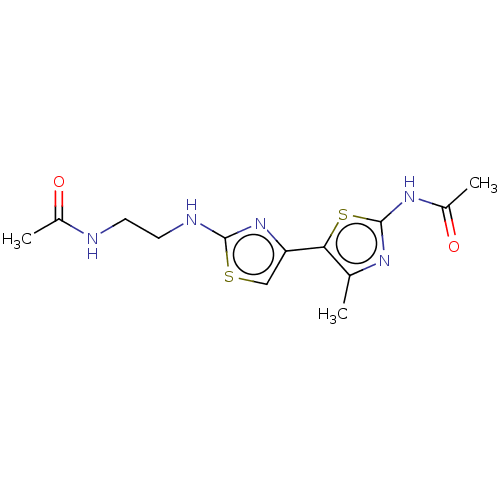 (US8802861, 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128369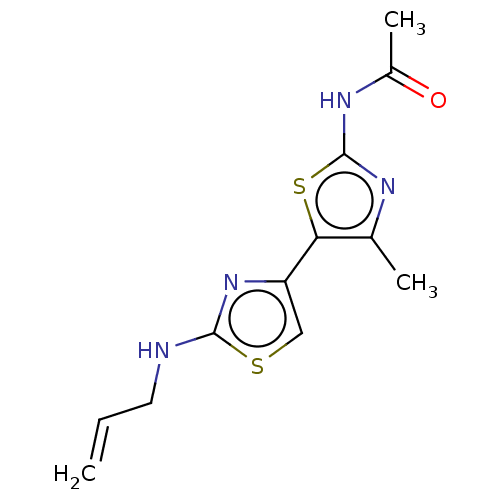 (US8802861, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128373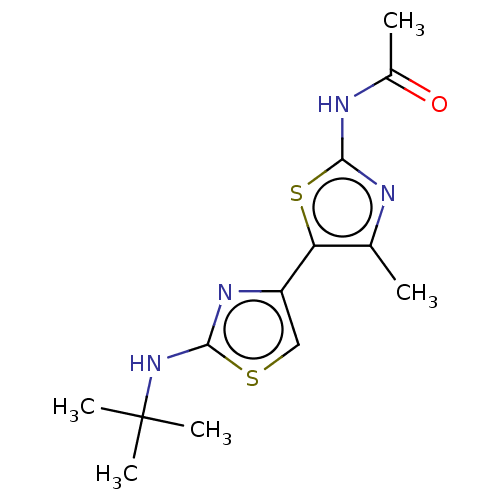 (US8802861, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128375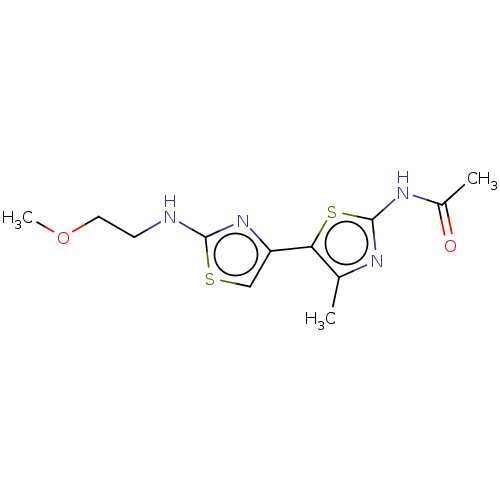 (US8802861, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128380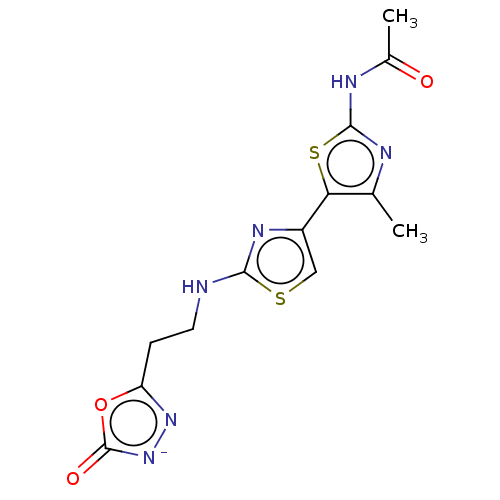 (US8802861, 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128371 (US8802861, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128378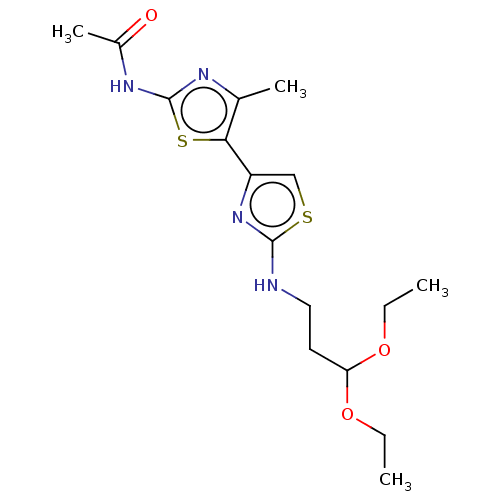 (US8802861, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 612 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM128368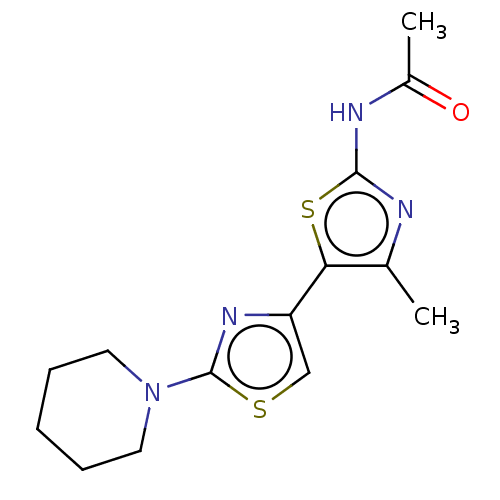 (US8802861, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA US Patent | Assay Description The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay.The assa... | US Patent US8802861 (2014) BindingDB Entry DOI: 10.7270/Q2HD7TCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |