 Found 340 hits with Last Name = 'savy' and Initial = 'p'
Found 340 hits with Last Name = 'savy' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315314
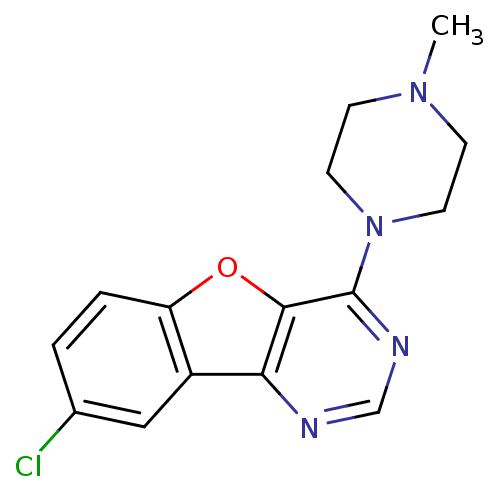
(8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...)Show InChI InChI=1S/C15H15ClN4O/c1-19-4-6-20(7-5-19)15-14-13(17-9-18-15)11-8-10(16)2-3-12(11)21-14/h2-3,8-9H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine human recombinant histamine H4 receptor |
Bioorg Med Chem Lett 20: 2516-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.097
BindingDB Entry DOI: 10.7270/Q2CN74V3 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine human recombinant histamine H4 receptor |
Bioorg Med Chem Lett 20: 2516-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.097
BindingDB Entry DOI: 10.7270/Q2CN74V3 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine human recombinant histamine H4 receptor |
Bioorg Med Chem Lett 20: 2516-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.097
BindingDB Entry DOI: 10.7270/Q2CN74V3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394918
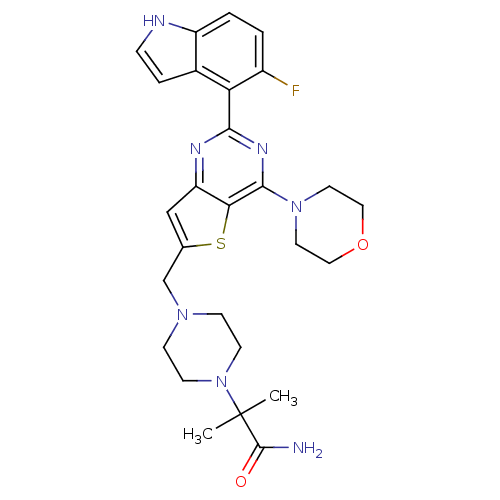
(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394917
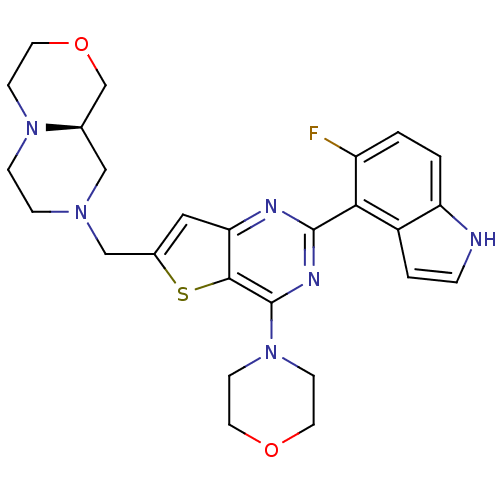
(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394916
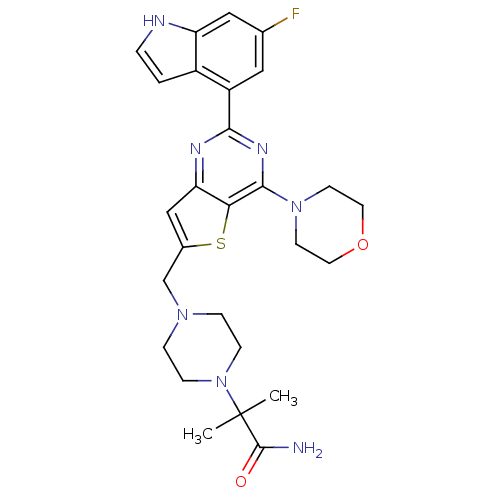
(CHEMBL2165506)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(F)cc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-7-5-33(6-8-35)16-18-15-22-23(38-18)25(34-9-11-37-12-10-34)32-24(31-22)20-13-17(28)14-21-19(20)3-4-30-21/h3-4,13-15,30H,5-12,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394910
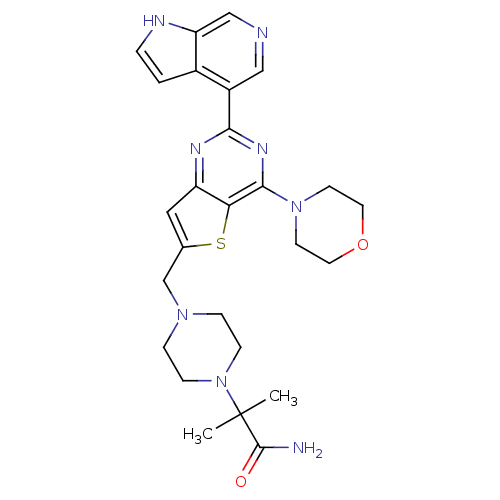
(CHEMBL2165512)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cncc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-7-5-32(6-8-34)16-17-13-20-22(37-17)24(33-9-11-36-12-10-33)31-23(30-20)19-14-28-15-21-18(19)3-4-29-21/h3-4,13-15,29H,5-12,16H2,1-2H3,(H2,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414477
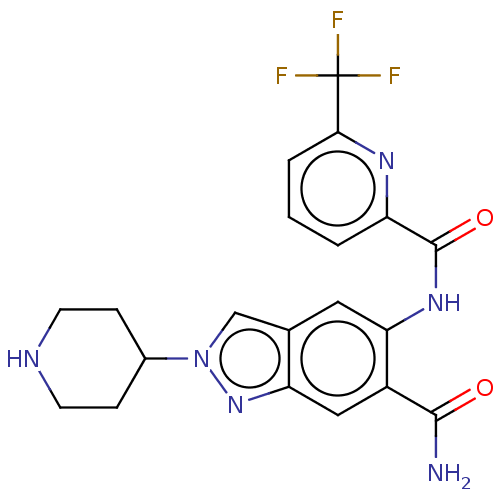
(2-(Piperidin-4-yl)-5-({[6-(trifluoromethyl)pyridin...)Show SMILES NC(=O)c1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCNCC1 Show InChI InChI=1S/C20H19F3N6O2/c21-20(22,23)17-3-1-2-14(26-17)19(31)27-16-8-11-10-29(12-4-6-25-7-5-12)28-15(11)9-13(16)18(24)30/h1-3,8-10,12,25H,4-7H2,(H2,24,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414436
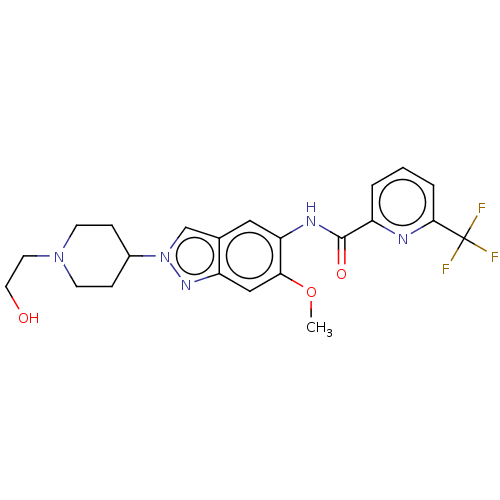
(N-{2-[1-(2-Hydroxyethyl)piperidin-4-yl]-6-methoxy-...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(CCO)CC1 Show InChI InChI=1S/C22H24F3N5O3/c1-33-19-12-17-14(13-30(28-17)15-5-7-29(8-6-15)9-10-31)11-18(19)27-21(32)16-3-2-4-20(26-16)22(23,24)25/h2-4,11-13,15,31H,5-10H2,1H3,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414444
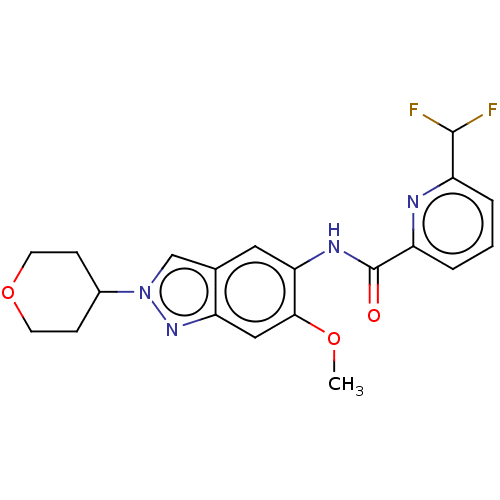
(6-(Difluoromethyl)-N-[6-methoxy-2-(tetrahydro-2H-p...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)F)C1CCOCC1 Show InChI InChI=1S/C20H20F2N4O3/c1-28-18-10-16-12(11-26(25-16)13-5-7-29-8-6-13)9-17(18)24-20(27)15-4-2-3-14(23-15)19(21)22/h2-4,9-11,13,19H,5-8H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414476
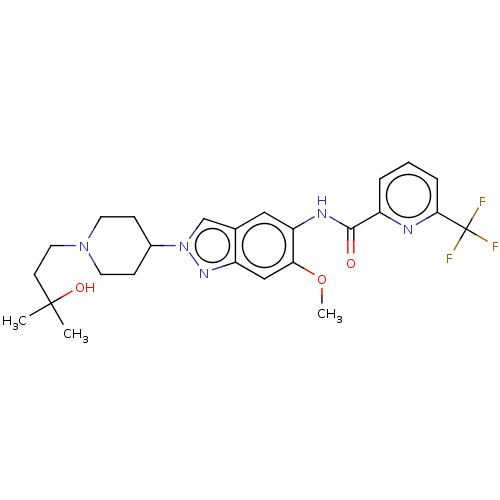
(N-{2-[1-(3-Hydroxy-3-methylbutyl)piperidin-4-yl]-6...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(CCC(C)(C)O)CC1 Show InChI InChI=1S/C25H30F3N5O3/c1-24(2,35)9-12-32-10-7-17(8-11-32)33-15-16-13-20(21(36-3)14-19(16)31-33)30-23(34)18-5-4-6-22(29-18)25(26,27)28/h4-6,13-15,17,35H,7-12H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414432
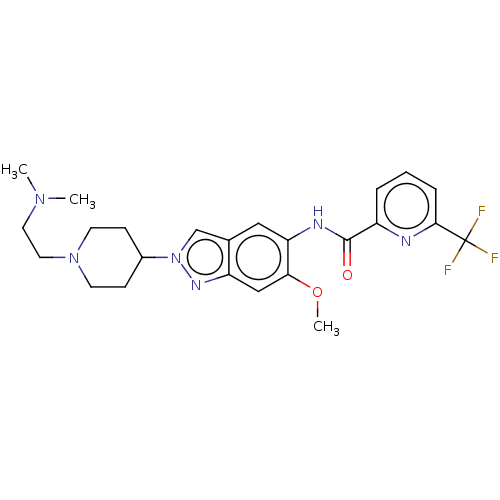
(N-(2-{1-[2-(Dimethylamino)ethyl]piperidin-4-yl}-6-...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(CCN(C)C)CC1 Show InChI InChI=1S/C24H29F3N6O2/c1-31(2)11-12-32-9-7-17(8-10-32)33-15-16-13-20(21(35-3)14-19(16)30-33)29-23(34)18-5-4-6-22(28-18)24(25,26)27/h4-6,13-15,17H,7-12H2,1-3H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315348
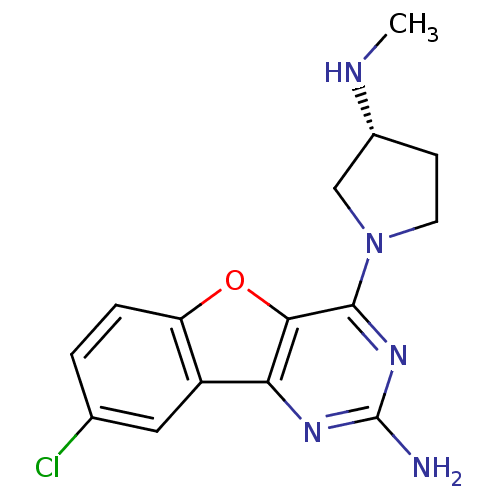
((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3oc12 |r| Show InChI InChI=1S/C15H16ClN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 20: 2516-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.097
BindingDB Entry DOI: 10.7270/Q2CN74V3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414479
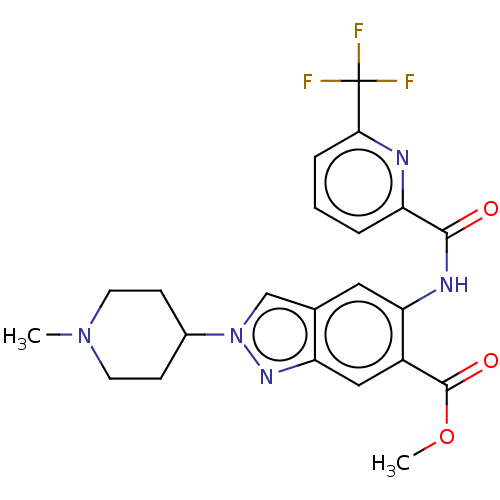
(Methyl 2-(1-methylpiperidin-4-yl)-5-({[6-(trifluor...)Show SMILES COC(=O)c1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(C)CC1 Show InChI InChI=1S/C22H22F3N5O3/c1-29-8-6-14(7-9-29)30-12-13-10-18(15(21(32)33-2)11-17(13)28-30)27-20(31)16-4-3-5-19(26-16)22(23,24)25/h3-5,10-12,14H,6-9H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414423
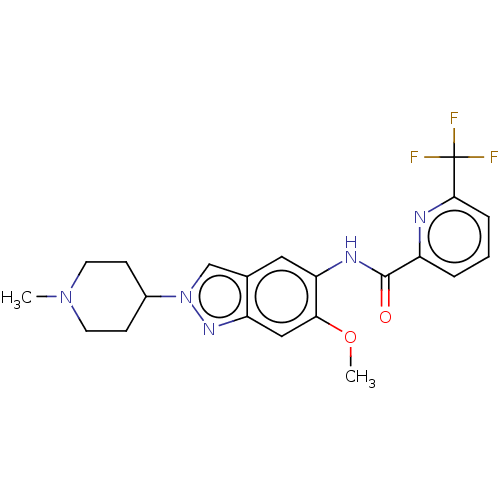
(N-[6-Methoxy-2-(1-methylpiperidin-4-yl)-2H-indazol...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(C)CC1 Show InChI InChI=1S/C21H22F3N5O2/c1-28-8-6-14(7-9-28)29-12-13-10-17(18(31-2)11-16(13)27-29)26-20(30)15-4-3-5-19(25-15)21(22,23)24/h3-5,10-12,14H,6-9H2,1-2H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396630
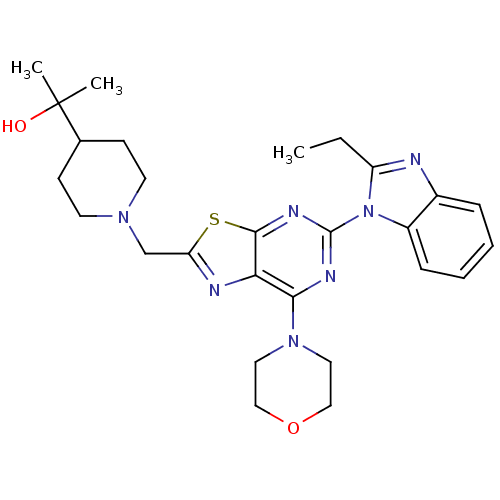
(CHEMBL2171946)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)sc2n1 Show InChI InChI=1S/C27H35N7O2S/c1-4-21-28-19-7-5-6-8-20(19)34(21)26-30-24(33-13-15-36-16-14-33)23-25(31-26)37-22(29-23)17-32-11-9-18(10-12-32)27(2,3)35/h5-8,18,35H,4,9-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396629
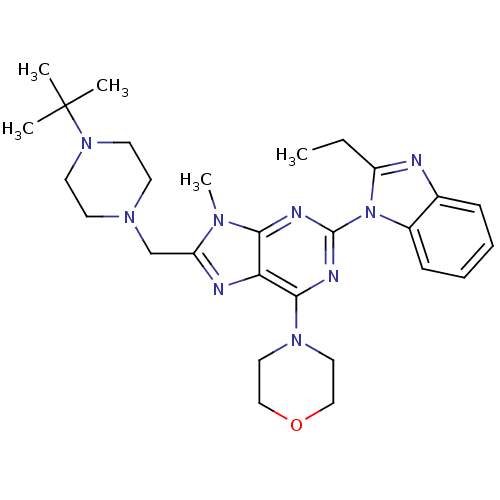
(CHEMBL2171940)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCN(CC3)C(C)(C)C)n(C)c2n1 Show InChI InChI=1S/C28H39N9O/c1-6-22-29-20-9-7-8-10-21(20)37(22)27-31-25-24(26(32-27)35-15-17-38-18-16-35)30-23(33(25)5)19-34-11-13-36(14-12-34)28(2,3)4/h7-10H,6,11-19H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414421
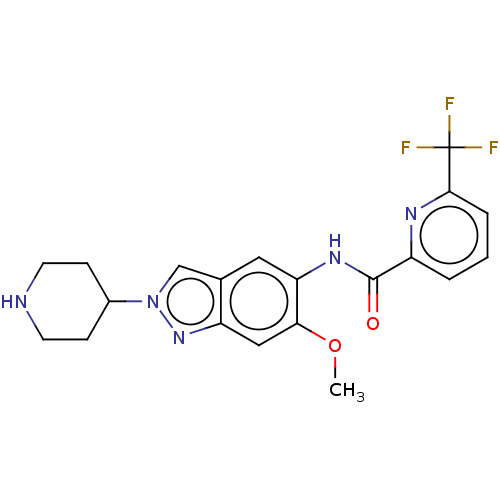
(N-[6-Methoxy-2-(piperidin-4-yl)-2H-indazol-5-yl]-6...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCNCC1 Show InChI InChI=1S/C20H20F3N5O2/c1-30-17-10-15-12(11-28(27-15)13-5-7-24-8-6-13)9-16(17)26-19(29)14-3-2-4-18(25-14)20(21,22)23/h2-4,9-11,13,24H,5-8H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394913

(CHEMBL2165509)Show SMILES Cc1cc2c(cccc2[nH]1)-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)C(C)(C)C(N)=O)cc2n1 Show InChI InChI=1S/C28H35N7O2S/c1-18-15-21-20(5-4-6-22(21)30-18)25-31-23-16-19(38-24(23)26(32-25)34-11-13-37-14-12-34)17-33-7-9-35(10-8-33)28(2,3)27(29)36/h4-6,15-16,30H,7-14,17H2,1-3H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394920
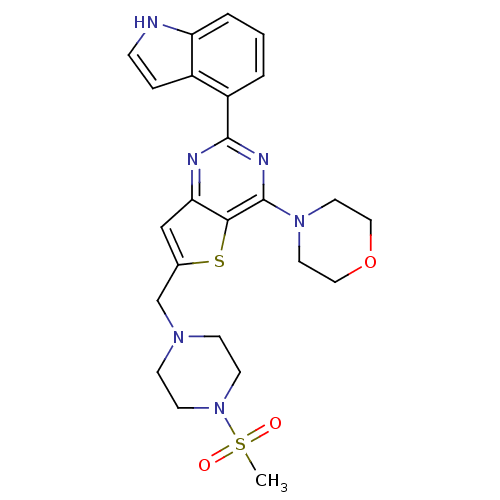
(CHEMBL2165672)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C24H28N6O3S2/c1-35(31,32)30-9-7-28(8-10-30)16-17-15-21-22(34-17)24(29-11-13-33-14-12-29)27-23(26-21)19-3-2-4-20-18(19)5-6-25-20/h2-6,15,25H,7-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394921
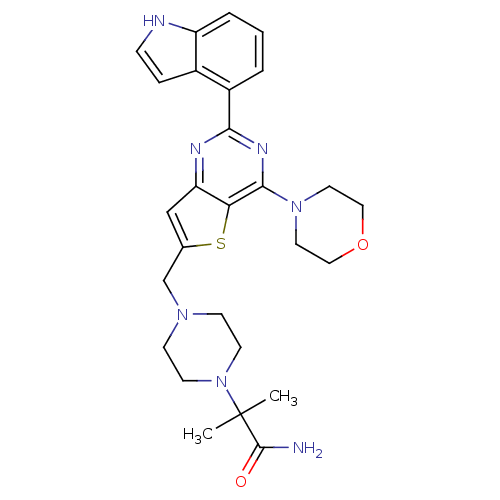
(CHEMBL2165671)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H33N7O2S/c1-27(2,26(28)35)34-10-8-32(9-11-34)17-18-16-22-23(37-18)25(33-12-14-36-15-13-33)31-24(30-22)20-4-3-5-21-19(20)6-7-29-21/h3-7,16,29H,8-15,17H2,1-2H3,(H2,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394908
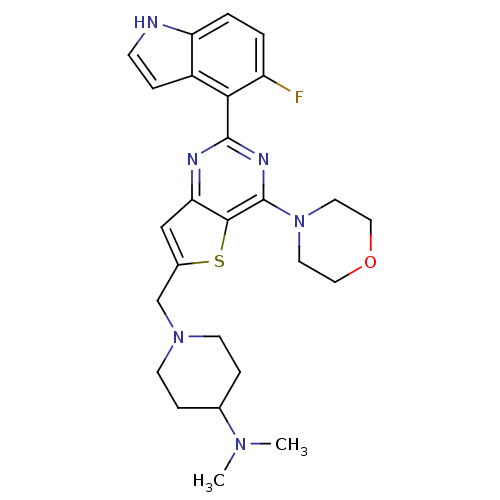
(CHEMBL2165666)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C26H31FN6OS/c1-31(2)17-6-9-32(10-7-17)16-18-15-22-24(35-18)26(33-11-13-34-14-12-33)30-25(29-22)23-19-5-8-28-21(19)4-3-20(23)27/h3-5,8,15,17,28H,6-7,9-14,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414445
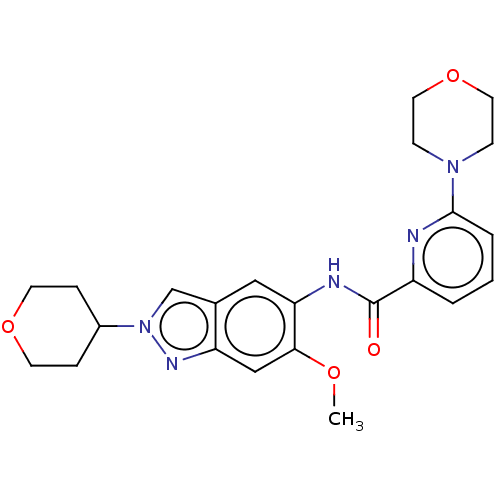
(N-[6-Methoxy-2-(tetrahydro-2H-pyran-4-yl)-2H-indaz...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)N1CCOCC1)C1CCOCC1 Show InChI InChI=1S/C23H27N5O4/c1-30-21-14-19-16(15-28(26-19)17-5-9-31-10-6-17)13-20(21)25-23(29)18-3-2-4-22(24-18)27-7-11-32-12-8-27/h2-4,13-15,17H,5-12H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414439
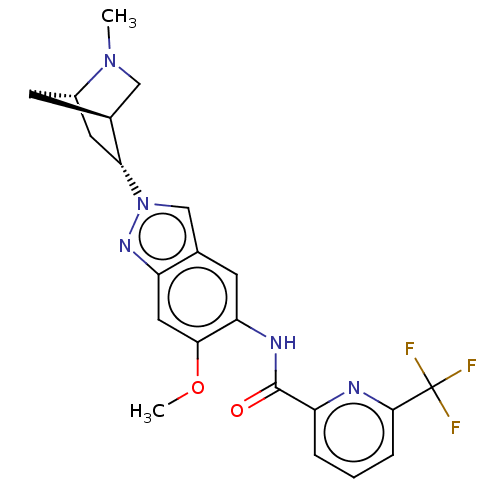
(US10435396, Example 18 | rel-N-{6-Methoxy-2-[(1S,4...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)[C@@H]1C[C@@H]2C[C@H]1CN2C |r| Show InChI InChI=1S/C22H22F3N5O2/c1-29-10-13-6-14(29)8-18(13)30-11-12-7-17(19(32-2)9-16(12)28-30)27-21(31)15-4-3-5-20(26-15)22(23,24)25/h3-5,7,9,11,13-14,18H,6,8,10H2,1-2H3,(H,27,31)/t13-,14-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414435
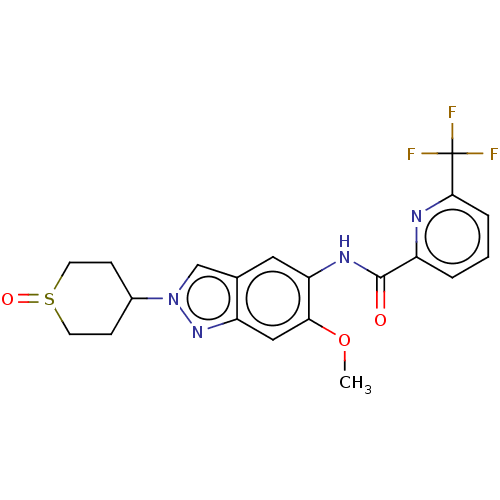
(US10435396, Example 12 | rac-N-[6-Methoxy-2-(1-oxi...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCS(=O)CC1 Show InChI InChI=1S/C20H19F3N4O3S/c1-30-17-10-15-12(11-27(26-15)13-5-7-31(29)8-6-13)9-16(17)25-19(28)14-3-2-4-18(24-14)20(21,22)23/h2-4,9-11,13H,5-8H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414428
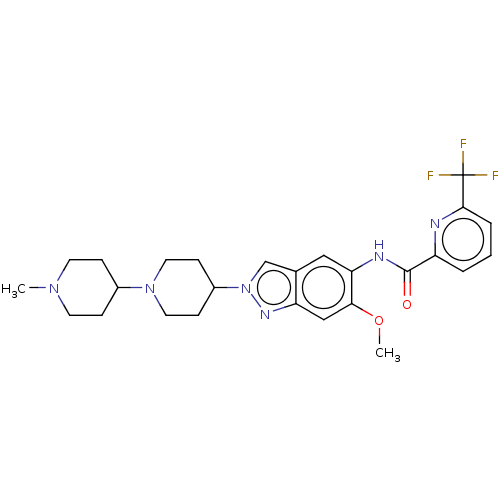
(N-[6-Methoxy-2-(1′-methyl-1,4′-bipiper...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(CC1)C1CCN(C)CC1 Show InChI InChI=1S/C26H31F3N6O2/c1-33-10-6-18(7-11-33)34-12-8-19(9-13-34)35-16-17-14-22(23(37-2)15-21(17)32-35)31-25(36)20-4-3-5-24(30-20)26(27,28)29/h3-5,14-16,18-19H,6-13H2,1-2H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414424
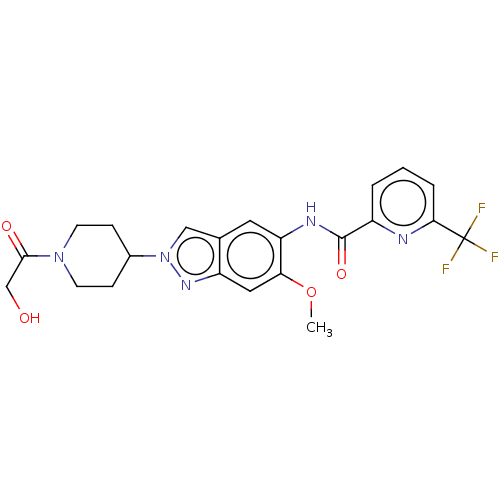
(N-[2-(1-Glycoloylpiperidin-4-yl)-6-methoxy-2H-inda...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(CC1)C(=O)CO Show InChI InChI=1S/C22H22F3N5O4/c1-34-18-10-16-13(11-30(28-16)14-5-7-29(8-6-14)20(32)12-31)9-17(18)27-21(33)15-3-2-4-19(26-15)22(23,24)25/h2-4,9-11,14,31H,5-8,12H2,1H3,(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396628
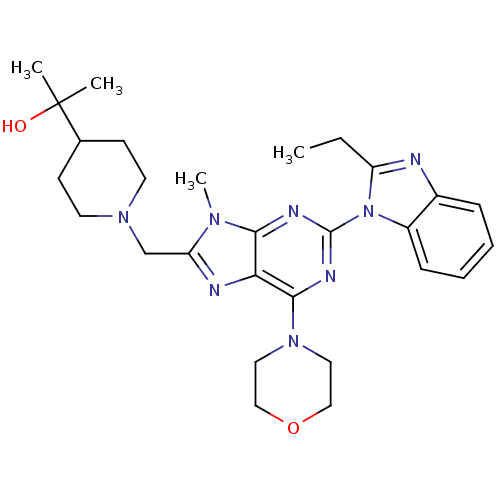
(CHEMBL2171944)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C28H38N8O2/c1-5-22-29-20-8-6-7-9-21(20)36(22)27-31-25-24(26(32-27)35-14-16-38-17-15-35)30-23(33(25)4)18-34-12-10-19(11-13-34)28(2,3)37/h6-9,19,37H,5,10-18H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414482
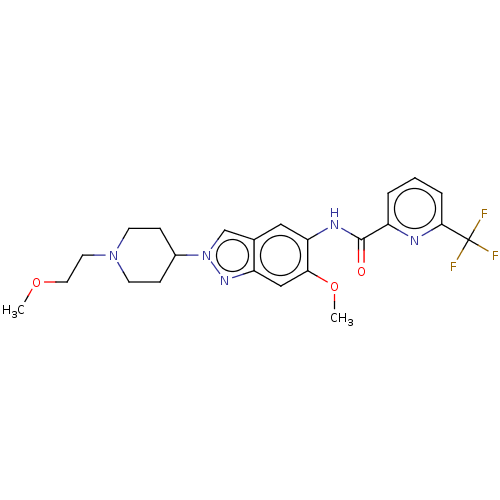
(N-{6-Methoxy-2-[1-(2-methoxyethyl)piperidin-4-yl]-...)Show SMILES COCCN1CCC(CC1)n1cc2cc(NC(=O)c3cccc(n3)C(F)(F)F)c(OC)cc2n1 Show InChI InChI=1S/C23H26F3N5O3/c1-33-11-10-30-8-6-16(7-9-30)31-14-15-12-19(20(34-2)13-18(15)29-31)28-22(32)17-4-3-5-21(27-17)23(24,25)26/h3-5,12-14,16H,6-11H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414481
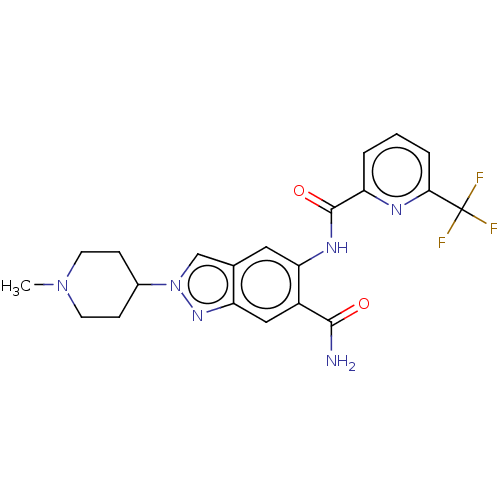
(2-(1-Methylpiperidin-4-yl)-5-({[6-(trifluoromethyl...)Show SMILES CN1CCC(CC1)n1cc2cc(NC(=O)c3cccc(n3)C(F)(F)F)c(cc2n1)C(N)=O Show InChI InChI=1S/C21H21F3N6O2/c1-29-7-5-13(6-8-29)30-11-12-9-17(14(19(25)31)10-16(12)28-30)27-20(32)15-3-2-4-18(26-15)21(22,23)24/h2-4,9-11,13H,5-8H2,1H3,(H2,25,31)(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396633
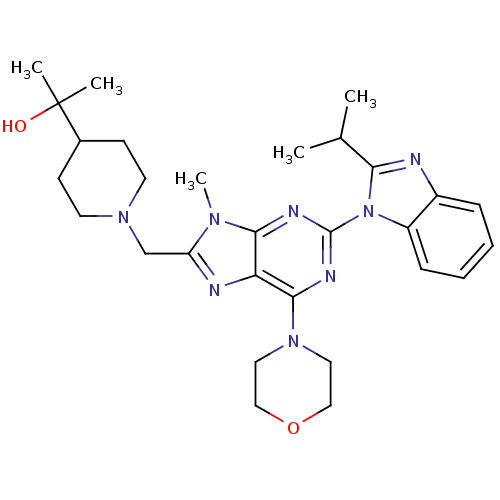
(CHEMBL2171952)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C29H40N8O2/c1-19(2)25-30-21-8-6-7-9-22(21)37(25)28-32-26-24(27(33-28)36-14-16-39-17-15-36)31-23(34(26)5)18-35-12-10-20(11-13-35)29(3,4)38/h6-9,19-20,38H,10-18H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396642
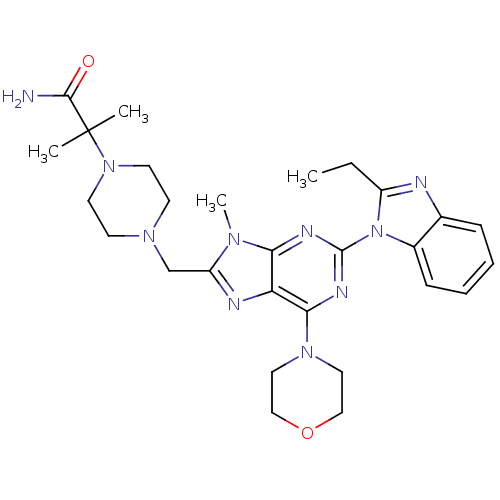
(CHEMBL2171939)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H38N10O2/c1-5-21-30-19-8-6-7-9-20(19)38(21)27-32-24-23(25(33-27)36-14-16-40-17-15-36)31-22(34(24)4)18-35-10-12-37(13-11-35)28(2,3)26(29)39/h6-9H,5,10-18H2,1-4H3,(H2,29,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396641

(CHEMBL2171942)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CC(C3)C3CCOCC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O2/c1-3-23-29-21-6-4-5-7-22(21)36(23)28-31-26-25(27(32-28)35-10-14-38-15-11-35)30-24(33(26)2)18-34-16-20(17-34)19-8-12-37-13-9-19/h4-7,19-20H,3,8-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396627
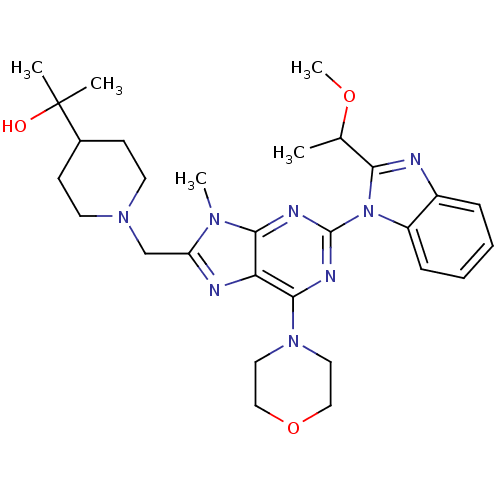
(CHEMBL2171949)Show SMILES COC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C29H40N8O3/c1-19(39-5)25-30-21-8-6-7-9-22(21)37(25)28-32-26-24(27(33-28)36-14-16-40-17-15-36)31-23(34(26)4)18-35-12-10-20(11-13-35)29(2,3)38/h6-9,19-20,38H,10-18H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394893
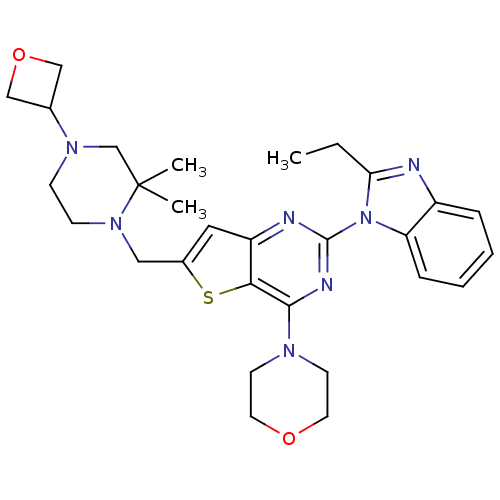
(CHEMBL2165502)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3(C)C)C3COC3)cc2n1 Show InChI InChI=1S/C29H37N7O2S/c1-4-25-30-22-7-5-6-8-24(22)36(25)28-31-23-15-21(39-26(23)27(32-28)33-11-13-37-14-12-33)16-35-10-9-34(19-29(35,2)3)20-17-38-18-20/h5-8,15,20H,4,9-14,16-19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394909
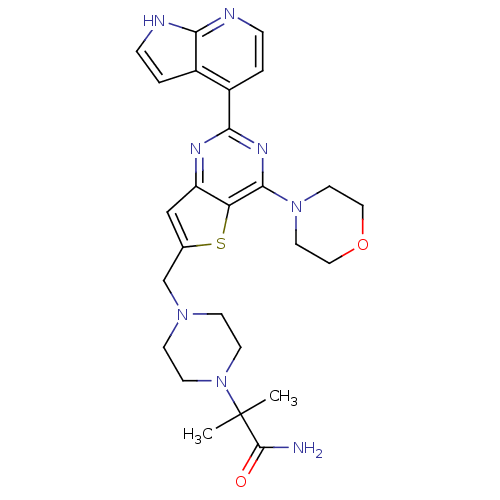
(CHEMBL2165513)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccnc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-9-7-32(8-10-34)16-17-15-20-21(37-17)24(33-11-13-36-14-12-33)31-23(30-20)19-4-6-29-22-18(19)3-5-28-22/h3-6,15H,7-14,16H2,1-2H3,(H2,27,35)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394903
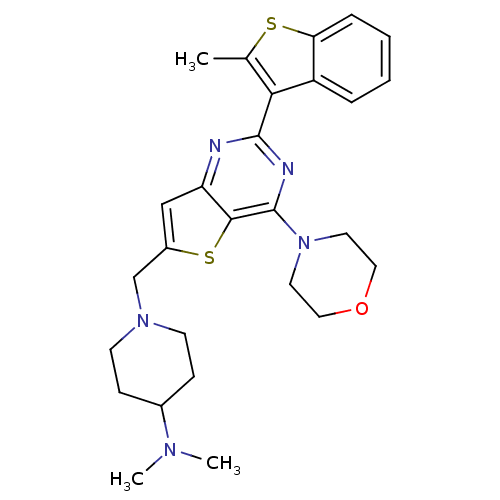
(CHEMBL2165492)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(C)sc3ccccc23)CC1 Show InChI InChI=1S/C27H33N5OS2/c1-18-24(21-6-4-5-7-23(21)34-18)26-28-22-16-20(17-31-10-8-19(9-11-31)30(2)3)35-25(22)27(29-26)32-12-14-33-15-13-32/h4-7,16,19H,8-15,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394897
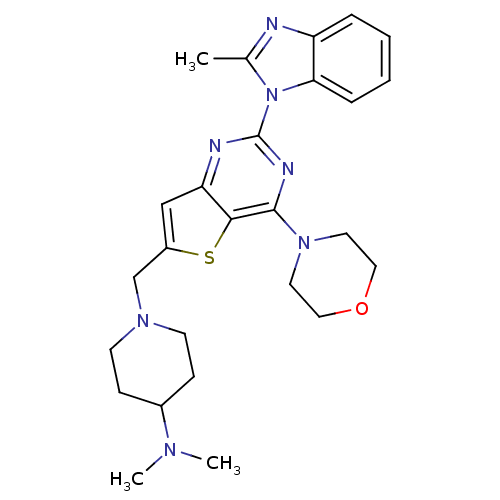
(CHEMBL2165498)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-n2c(C)nc3ccccc23)CC1 Show InChI InChI=1S/C26H33N7OS/c1-18-27-21-6-4-5-7-23(21)33(18)26-28-22-16-20(17-31-10-8-19(9-11-31)30(2)3)35-24(22)25(29-26)32-12-14-34-15-13-32/h4-7,16,19H,8-15,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396636
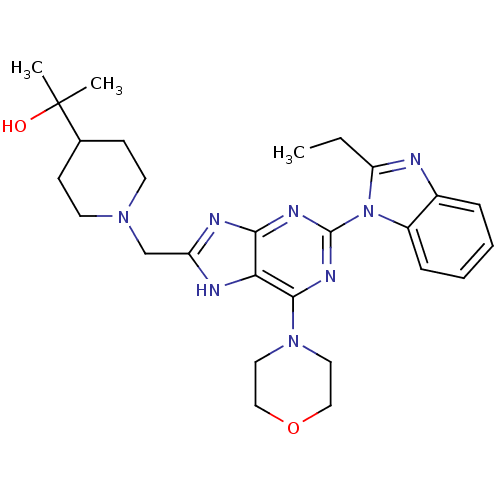
(CHEMBL2171943)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2[nH]c(CN3CCC(CC3)C(C)(C)O)nc2n1 Show InChI InChI=1S/C27H36N8O2/c1-4-22-28-19-7-5-6-8-20(19)35(22)26-31-24-23(25(32-26)34-13-15-37-16-14-34)29-21(30-24)17-33-11-9-18(10-12-33)27(2,3)36/h5-8,18,36H,4,9-17H2,1-3H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394918

(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50315348

((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c3cc(Cl)ccc3oc12 |r| Show InChI InChI=1S/C15H16ClN5O/c1-18-9-4-5-21(7-9)14-13-12(19-15(17)20-14)10-6-8(16)2-3-11(10)22-13/h2-3,6,9,18H,4-5,7H2,1H3,(H2,17,19,20)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 20: 2516-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.097
BindingDB Entry DOI: 10.7270/Q2CN74V3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414433
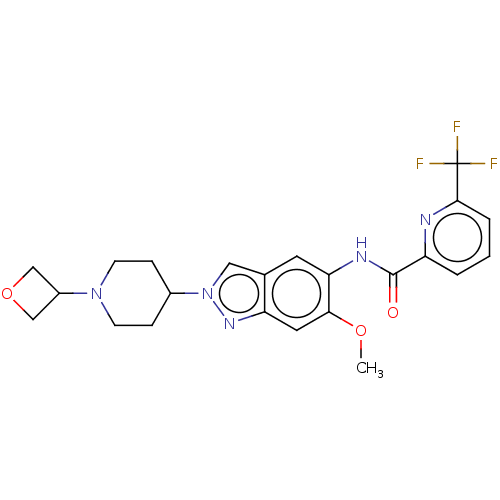
(N-{6-Methoxy-2-[1-(oxetan-3-yl)piperidin-4-yl]-2H-...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCN(CC1)C1COC1 Show InChI InChI=1S/C23H24F3N5O3/c1-33-20-10-18-14(11-31(29-18)15-5-7-30(8-6-15)16-12-34-13-16)9-19(20)28-22(32)17-3-2-4-21(27-17)23(24,25)26/h2-4,9-11,15-16H,5-8,12-13H2,1H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414442
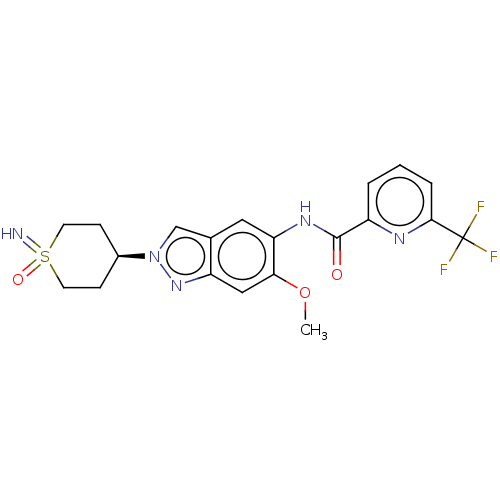
(N-[2-(1-Imino-1-oxidohexahydro-1λ4-thiopyran-...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)[C@H]1CCS(=N)(=O)CC1 |r| Show InChI InChI=1S/C20H20F3N5O3S/c1-31-17-10-15-12(11-28(27-15)13-5-7-32(24,30)8-6-13)9-16(17)26-19(29)14-3-2-4-18(25-14)20(21,22)23/h2-4,9-11,13,24H,5-8H2,1H3,(H,26,29)/t13-,32? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414457
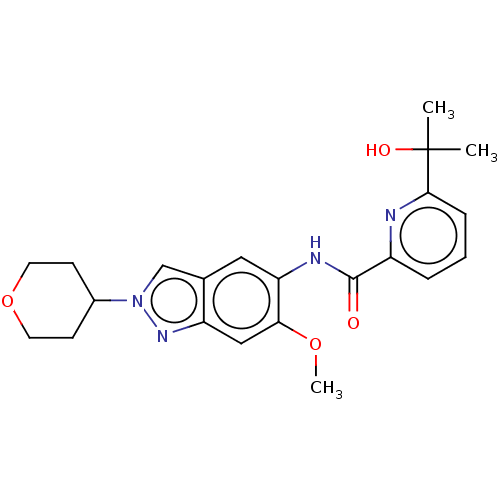
(6-(2-Hydroxypropan-2-yl)-N-[6-methoxy-2-(tetrahydr...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(C)(C)O)C1CCOCC1 Show InChI InChI=1S/C22H26N4O4/c1-22(2,28)20-6-4-5-16(23-20)21(27)24-18-11-14-13-26(15-7-9-30-10-8-15)25-17(14)12-19(18)29-3/h4-6,11-13,15,28H,7-10H2,1-3H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396634
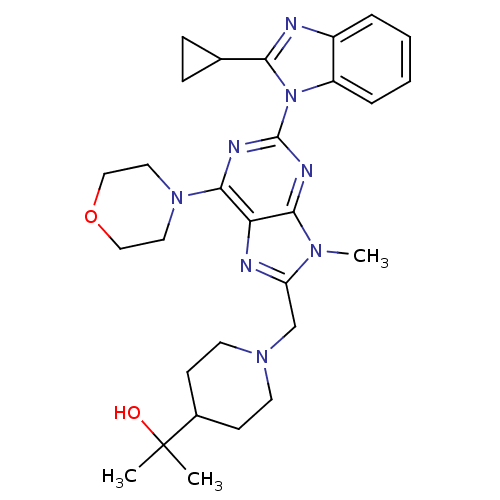
(CHEMBL2171950)Show SMILES Cn1c(CN2CCC(CC2)C(C)(C)O)nc2c(nc(nc12)-n1c(nc2ccccc12)C1CC1)N1CCOCC1 Show InChI InChI=1S/C29H38N8O2/c1-29(2,38)20-10-12-35(13-11-20)18-23-31-24-26(34(23)3)32-28(33-27(24)36-14-16-39-17-15-36)37-22-7-5-4-6-21(22)30-25(37)19-8-9-19/h4-7,19-20,38H,8-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396640
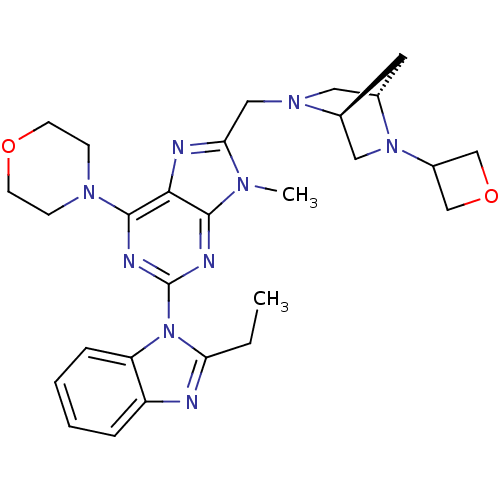
(CHEMBL2171941)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3C[C@@H]4C[C@H]3CN4C3COC3)n(C)c2n1 |r| Show InChI InChI=1S/C28H35N9O2/c1-3-23-29-21-6-4-5-7-22(21)37(23)28-31-26-25(27(32-28)34-8-10-38-11-9-34)30-24(33(26)2)15-35-13-19-12-18(35)14-36(19)20-16-39-17-20/h4-7,18-20H,3,8-17H2,1-2H3/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as inhibition of PIP3 production for 30 mins by fluorescence polarization assay |
J Med Chem 55: 7686-95 (2012)
Article DOI: 10.1021/jm300717c
BindingDB Entry DOI: 10.7270/Q2DB8302 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM414434
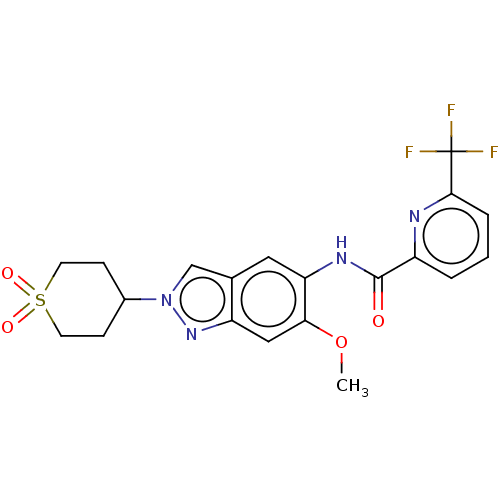
(N-[2-(1,1-Dioxidotetrahydro-2H-thiopyran-4-yl)-6-m...)Show SMILES COc1cc2nn(cc2cc1NC(=O)c1cccc(n1)C(F)(F)F)C1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H19F3N4O4S/c1-31-17-10-15-12(11-27(26-15)13-5-7-32(29,30)8-6-13)9-16(17)25-19(28)14-3-2-4-18(24-14)20(21,22)23/h2-4,9-11,13H,5-8H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft
US Patent
| Assay Description
For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... |
US Patent US10435396 (2019)
BindingDB Entry DOI: 10.7270/Q28C9ZMZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data