

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278990 (CHEMBL4164524) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278986 (CHEMBL4161860) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278968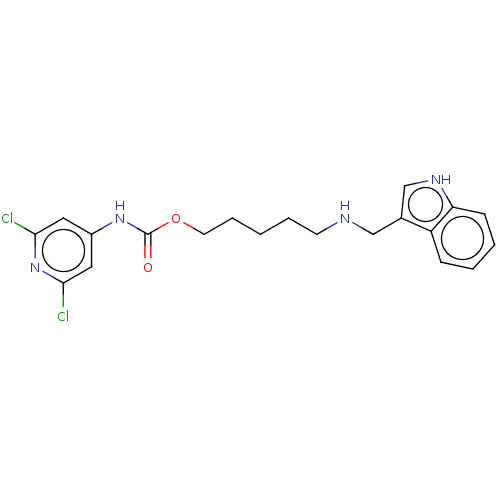 (CHEMBL4164186) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564589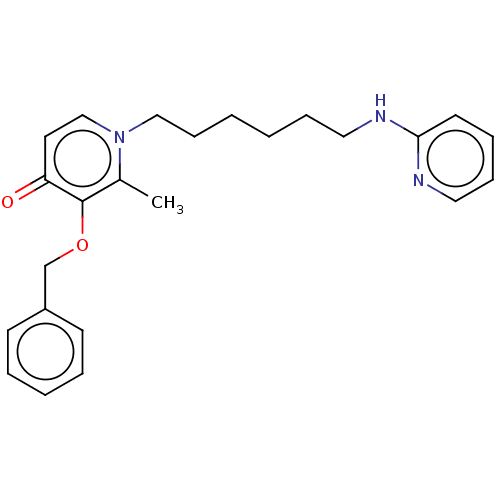 (CHEMBL4783436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278987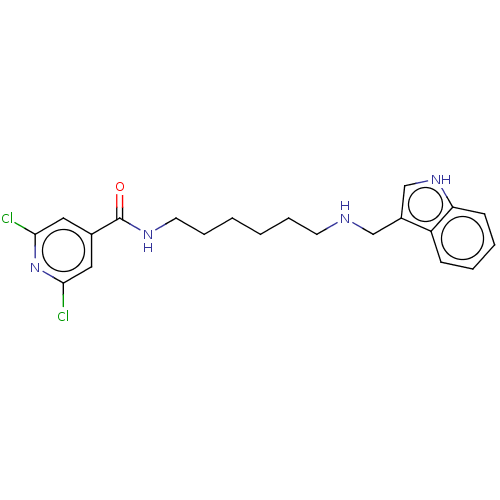 (CHEMBL4165299) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564591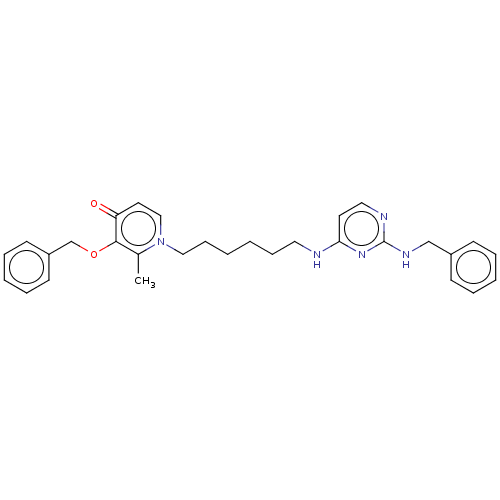 (CHEMBL4799735) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278969 (CHEMBL4160790) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278984 (CHEMBL4168710) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278970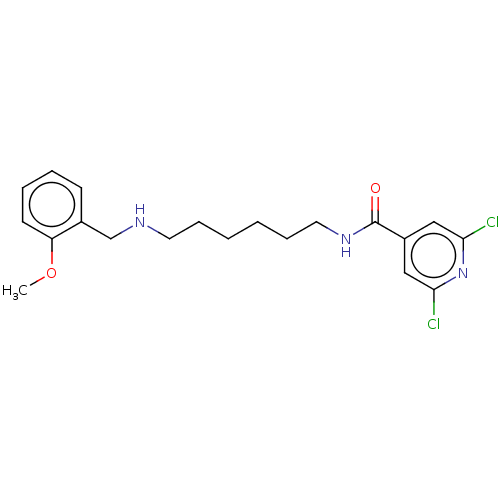 (CHEMBL4175914) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA nucleotidylexotransferase (Homo sapiens (Human)) | BDBM50442248 (CHEMBL2441983) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Non-competitive inhibition of human TdT using 3'-OH as substrate | J Med Chem 56: 7431-41 (2013) Article DOI: 10.1021/jm4010187 BindingDB Entry DOI: 10.7270/Q2H70H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278983 (CHEMBL4172510) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA nucleotidylexotransferase (Homo sapiens (Human)) | BDBM50442215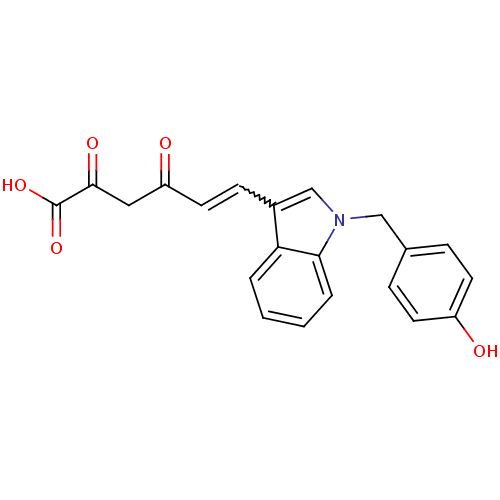 (CHEMBL2441974) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Competitive inhibition of human TdT using TTP as substrate | J Med Chem 56: 7431-41 (2013) Article DOI: 10.1021/jm4010187 BindingDB Entry DOI: 10.7270/Q2H70H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564588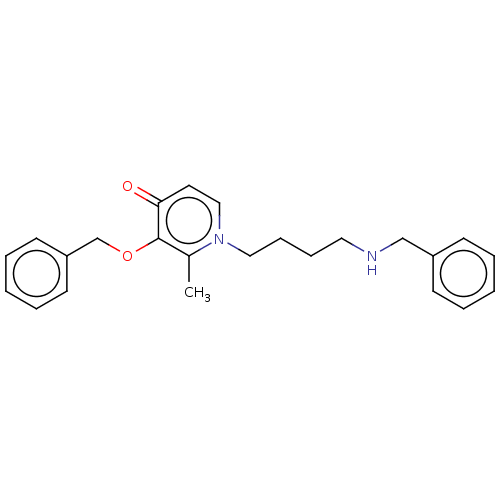 (CHEMBL4785902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA nucleotidylexotransferase (Homo sapiens (Human)) | BDBM50442248 (CHEMBL2441983) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Competitive inhibition of human TdT using TTP as substrate | J Med Chem 56: 7431-41 (2013) Article DOI: 10.1021/jm4010187 BindingDB Entry DOI: 10.7270/Q2H70H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA nucleotidylexotransferase (Homo sapiens (Human)) | BDBM50442215 (CHEMBL2441974) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Non-competitive inhibition of human TdT using 3'-OH as substrate | J Med Chem 56: 7431-41 (2013) Article DOI: 10.1021/jm4010187 BindingDB Entry DOI: 10.7270/Q2H70H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50564584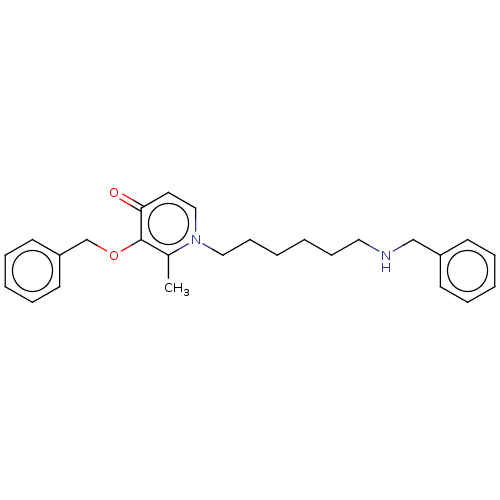 (CHEMBL4791565) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 788 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564585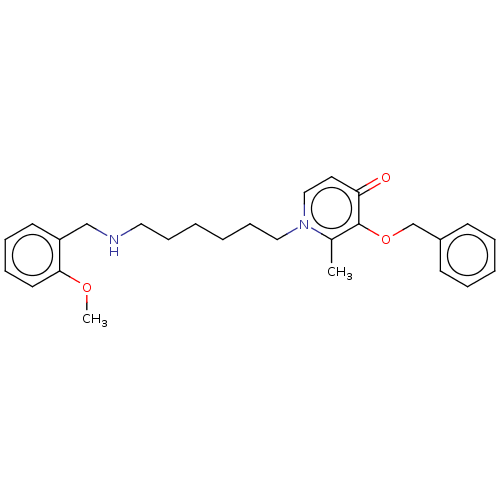 (CHEMBL4780976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564592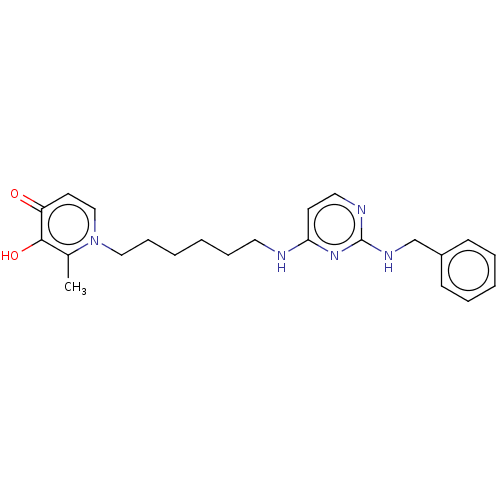 (CHEMBL4798261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564590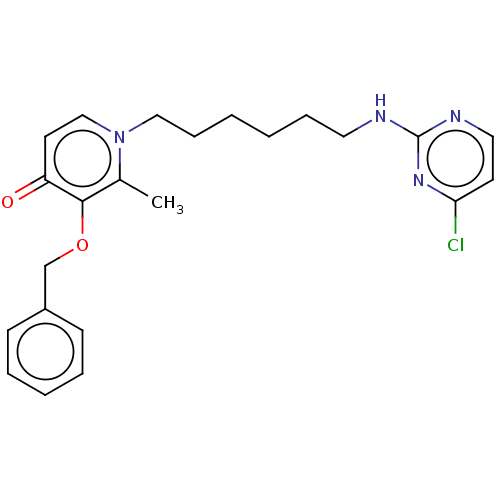 (CHEMBL4778716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50564587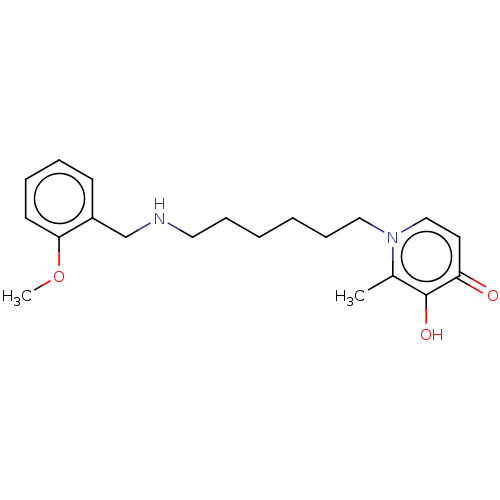 (CHEMBL4782376) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50564586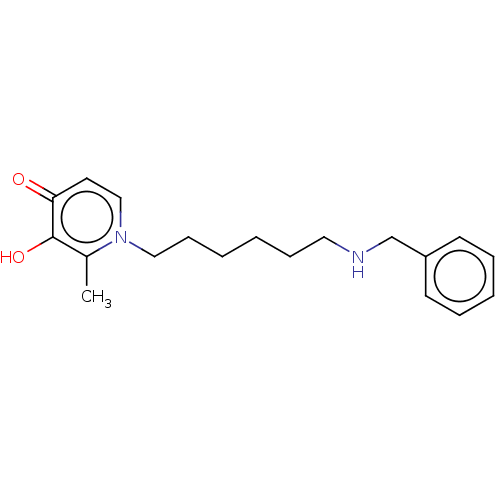 (CHEMBL4781943) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA nucleotidylexotransferase (Homo sapiens (Human)) | BDBM50442209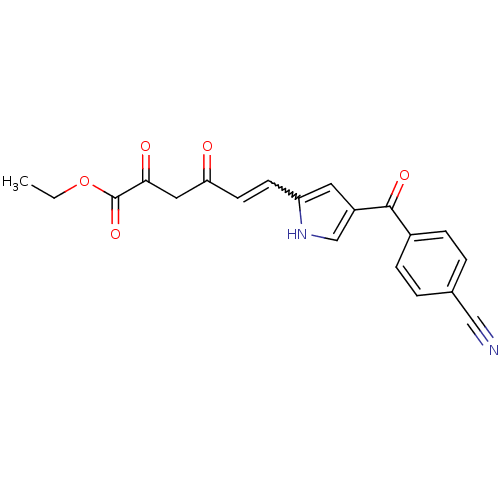 (CHEMBL2441980) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Competitive inhibition of human TdT using TTP as substrate | J Med Chem 56: 7431-41 (2013) Article DOI: 10.1021/jm4010187 BindingDB Entry DOI: 10.7270/Q2H70H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA nucleotidylexotransferase (Homo sapiens (Human)) | BDBM50442209 (CHEMBL2441980) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Non-competitive inhibition of human TdT using 3'-OH as substrate | J Med Chem 56: 7431-41 (2013) Article DOI: 10.1021/jm4010187 BindingDB Entry DOI: 10.7270/Q2H70H8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564584 (CHEMBL4791565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine BChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50564585 (CHEMBL4780976) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine as substrate measured for 0.5 to 1.5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112350 BindingDB Entry DOI: 10.7270/Q25X2DP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227314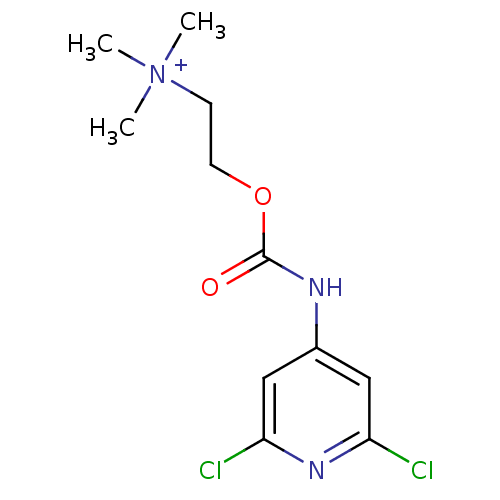 (2-(2,6-dichloropyridin-4-ylcarbamoyloxy)-N,N,N-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227319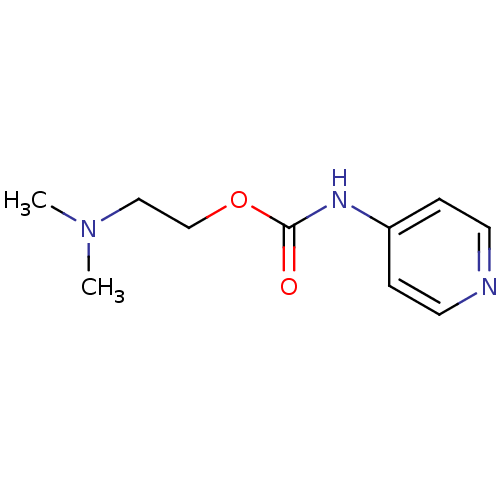 (2-(dimethylamino)ethyl pyridin-4-ylcarbamate | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227314 (2-(2,6-dichloropyridin-4-ylcarbamoyloxy)-N,N,N-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227318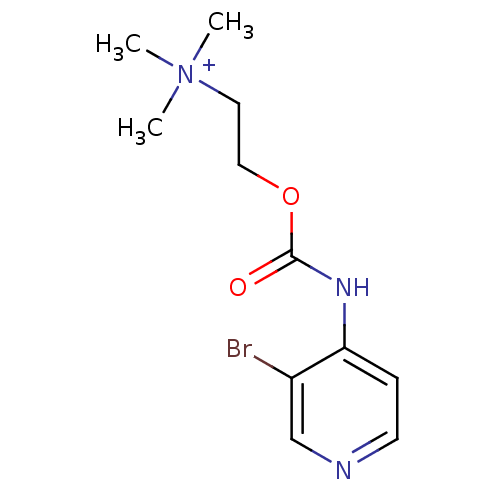 (2-(3-bromopyridin-4-ylcarbamoyloxy)-N,N,N-trimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227315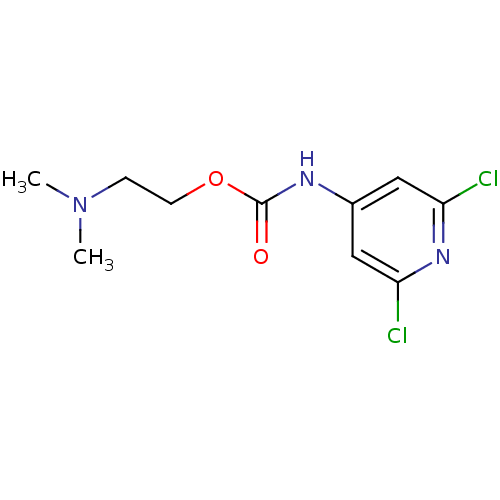 (2-(dimethylamino)ethyl 2,6-dichloropyridin-4-ylcar...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227312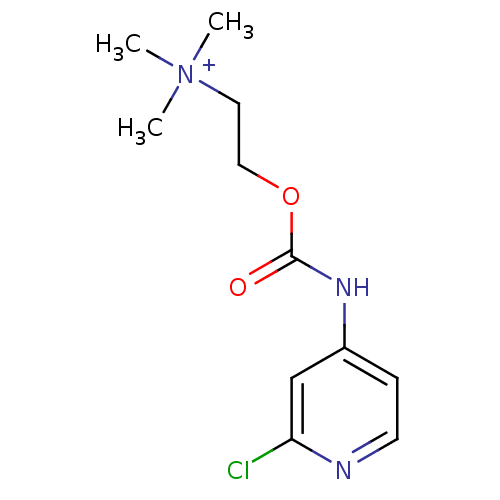 (2-(2-chloropyridin-4-ylcarbamoyloxy)-N,N,N-trimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227313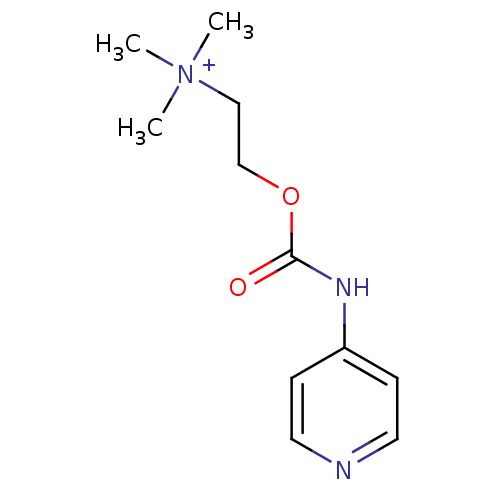 (CHEMBL254299 | N,N,N-trimethyl-2-(pyridin-4-ylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227312 (2-(2-chloropyridin-4-ylcarbamoyloxy)-N,N,N-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227315 (2-(dimethylamino)ethyl 2,6-dichloropyridin-4-ylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227316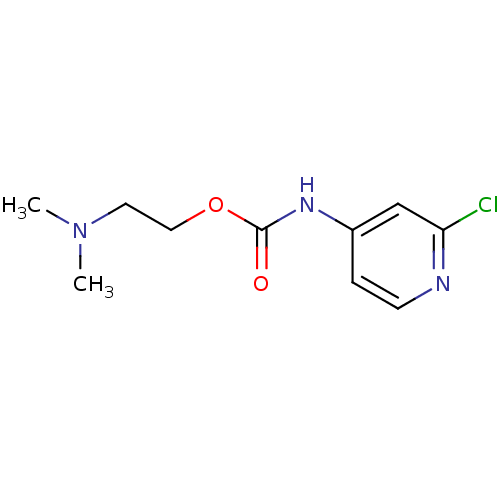 (2-(dimethylamino)ethyl 2-chloropyridin-4-ylcarbama...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227317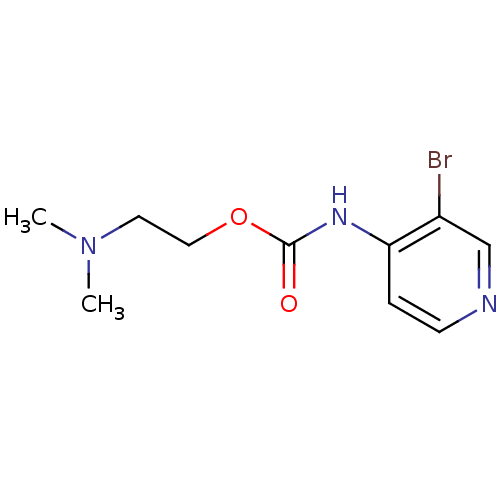 (2-(dimethylamino)ethyl 3-bromopyridin-4-ylcarbamat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227318 (2-(3-bromopyridin-4-ylcarbamoyloxy)-N,N,N-trimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227319 (2-(dimethylamino)ethyl pyridin-4-ylcarbamate | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50227317 (2-(dimethylamino)ethyl 3-bromopyridin-4-ylcarbamat...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227316 (2-(dimethylamino)ethyl 2-chloropyridin-4-ylcarbama...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50227313 (CHEMBL254299 | N,N,N-trimethyl-2-(pyridin-4-ylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase | Bioorg Med Chem Lett 18: 309-12 (2008) Article DOI: 10.1016/j.bmcl.2007.10.077 BindingDB Entry DOI: 10.7270/Q2PC3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50466629 (CHEMBL4280766) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay | J Med Chem 61: 10834-10859 (2018) Article DOI: 10.1021/acs.jmedchem.8b01497 BindingDB Entry DOI: 10.7270/Q29K4DWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50498306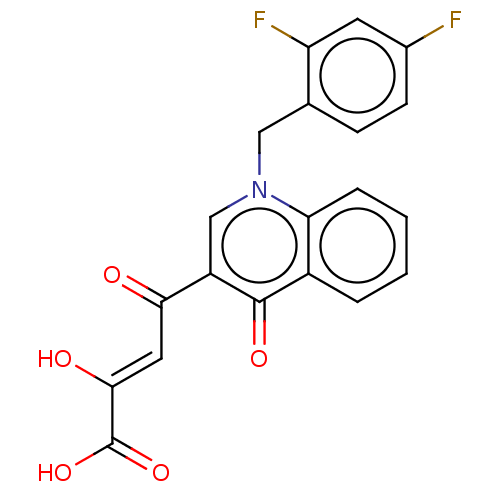 (CHEMBL3582062) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase assessed as inhibition of strand transfer activity using 32P-labeled DNA as substrate after 1 hr by gel-based assay in ... | J Med Chem 58: 4610-23 (2015) Article DOI: 10.1021/acs.jmedchem.5b00159 BindingDB Entry DOI: 10.7270/Q2QC06H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay | J Med Chem 62: 1330-1347 (2019) Article DOI: 10.1021/acs.jmedchem.8b01464 BindingDB Entry DOI: 10.7270/Q2CF9TKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479082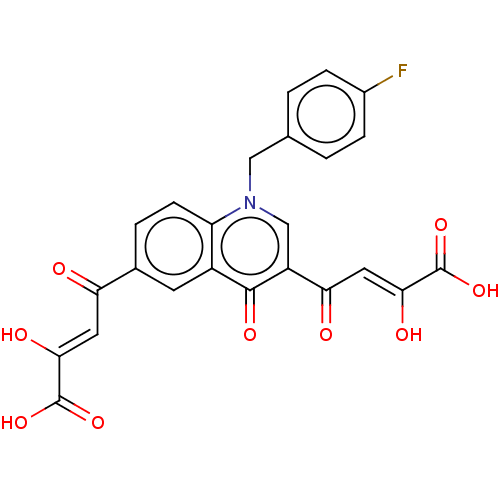 (CHEMBL497501) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase assessed as inhibition of strand transfer activity using 32P-labeled DNA as substrate after 1 hr by gel-based assay in ... | J Med Chem 58: 4610-23 (2015) Article DOI: 10.1021/acs.jmedchem.5b00159 BindingDB Entry DOI: 10.7270/Q2QC06H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50486615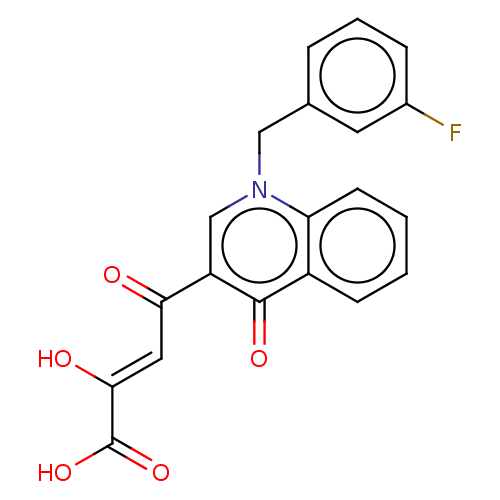 (CHEMBL2236599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase assessed as inhibition of strand transfer activity using 32P-labeled DNA as substrate after 1 hr by gel-based assay in ... | J Med Chem 58: 4610-23 (2015) Article DOI: 10.1021/acs.jmedchem.5b00159 BindingDB Entry DOI: 10.7270/Q2QC06H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50498303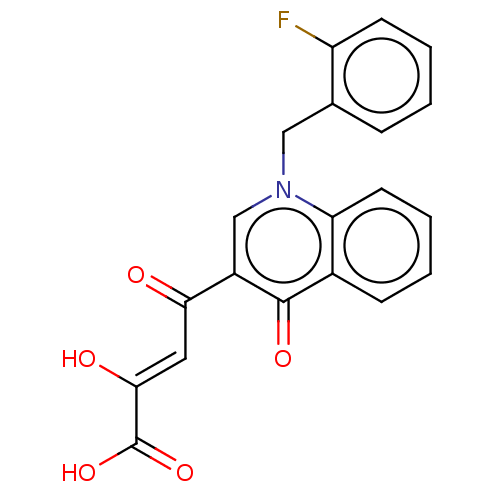 (CHEMBL3582057) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase assessed as inhibition of strand transfer activity using 32P-labeled DNA as substrate after 1 hr by gel-based assay in ... | J Med Chem 58: 4610-23 (2015) Article DOI: 10.1021/acs.jmedchem.5b00159 BindingDB Entry DOI: 10.7270/Q2QC06H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 629 total ) | Next | Last >> |