 Found 251 hits with Last Name = 'seo' and Initial = 'jh'
Found 251 hits with Last Name = 'seo' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50300459
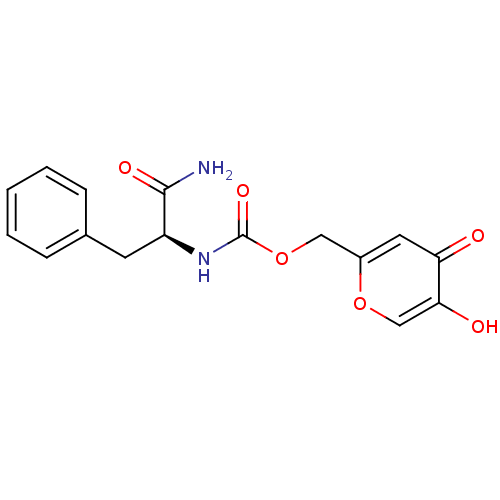
(CHEMBL573709 | Kojic acid-phenylalanine amide)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1cc(=O)c(O)co1 |r| Show InChI InChI=1S/C16H16N2O6/c17-15(21)12(6-10-4-2-1-3-5-10)18-16(22)24-8-11-7-13(19)14(20)9-23-11/h1-5,7,9,12,20H,6,8H2,(H2,17,21)(H,18,22)/t12-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPA |
Bioorg Med Chem Lett 19: 5586-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.041
BindingDB Entry DOI: 10.7270/Q2DF6R8S |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase assessed as oxidation of L-DOPA |
Bioorg Med Chem Lett 19: 5586-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.041
BindingDB Entry DOI: 10.7270/Q2DF6R8S |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391953
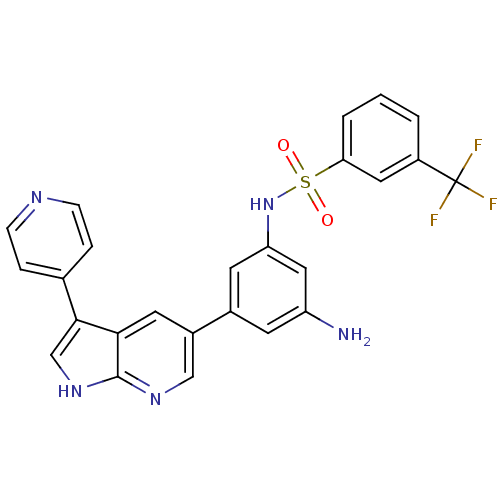
(CHEMBL2152288)Show SMILES Nc1cc(NS(=O)(=O)c2cccc(c2)C(F)(F)F)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H18F3N5O2S/c26-25(27,28)18-2-1-3-21(11-18)36(34,35)33-20-9-16(8-19(29)12-20)17-10-22-23(14-32-24(22)31-13-17)15-4-6-30-7-5-15/h1-14,33H,29H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391978
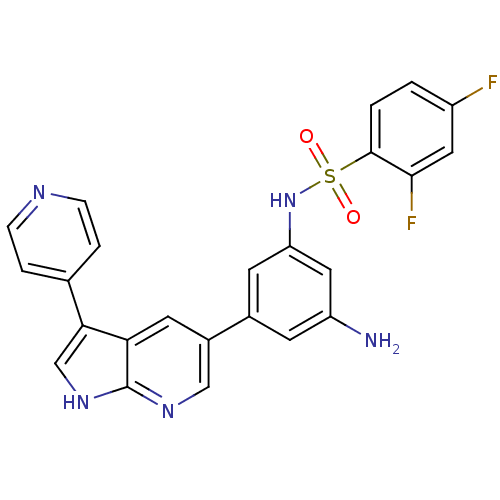
(CHEMBL2152287)Show SMILES Nc1cc(NS(=O)(=O)c2ccc(F)cc2F)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H17F2N5O2S/c25-17-1-2-23(22(26)10-17)34(32,33)31-19-8-15(7-18(27)11-19)16-9-20-21(13-30-24(20)29-12-16)14-3-5-28-6-4-14/h1-13,31H,27H2,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391977
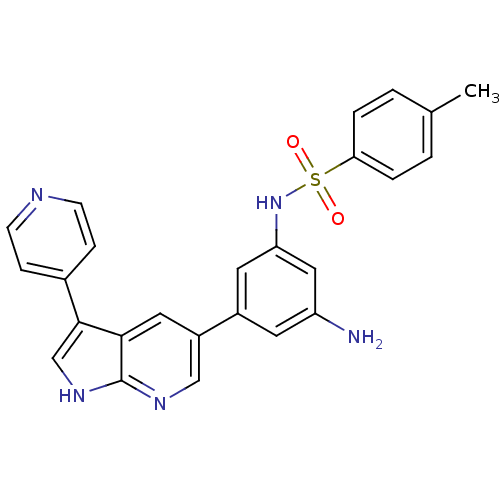
(CHEMBL2152286)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1cc(N)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H21N5O2S/c1-16-2-4-22(5-3-16)33(31,32)30-21-11-18(10-20(26)13-21)19-12-23-24(15-29-25(23)28-14-19)17-6-8-27-9-7-17/h2-15,30H,26H2,1H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391950
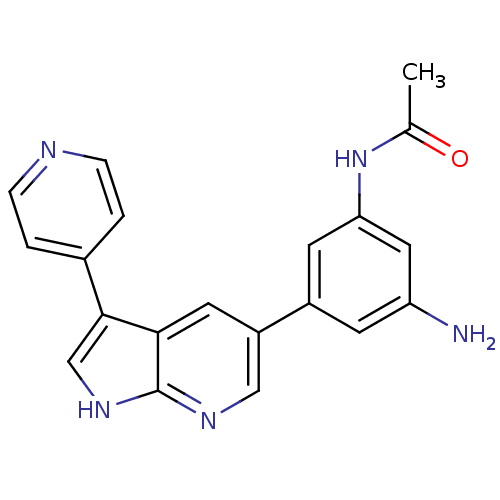
(CHEMBL2152289)Show SMILES CC(=O)Nc1cc(N)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C20H17N5O/c1-12(26)25-17-7-14(6-16(21)9-17)15-8-18-19(11-24-20(18)23-10-15)13-2-4-22-5-3-13/h2-11H,21H2,1H3,(H,23,24)(H,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50262085

(CHEMBL468397 | methyl 5-((3-(1H-benzo[d]imidazol-2...)Show SMILES COC(=O)C(CCCN(C)CCCc1nc2ccccc2[nH]1)(C(C)C)c1ccc(Br)cc1 Show InChI InChI=1S/C26H34BrN3O2/c1-19(2)26(25(31)32-4,20-12-14-21(27)15-13-20)16-8-18-30(3)17-7-11-24-28-22-9-5-6-10-23(22)29-24/h5-6,9-10,12-15,19H,7-8,11,16-18H2,1-4H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of T-type calcium channel subunit alpha-1G (unknown origin) expressed in HEK293 cells assessed as inactivation of channel current at holdi... |
Bioorg Med Chem Lett 24: 880-3 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.078
BindingDB Entry DOI: 10.7270/Q2C53PSG |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391974
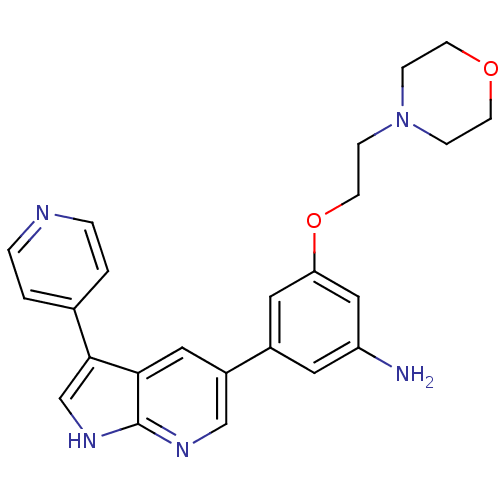
(CHEMBL2152283)Show SMILES Nc1cc(OCCN2CCOCC2)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H25N5O2/c25-20-11-18(12-21(14-20)31-10-7-29-5-8-30-9-6-29)19-13-22-23(16-28-24(22)27-15-19)17-1-3-26-4-2-17/h1-4,11-16H,5-10,25H2,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391976
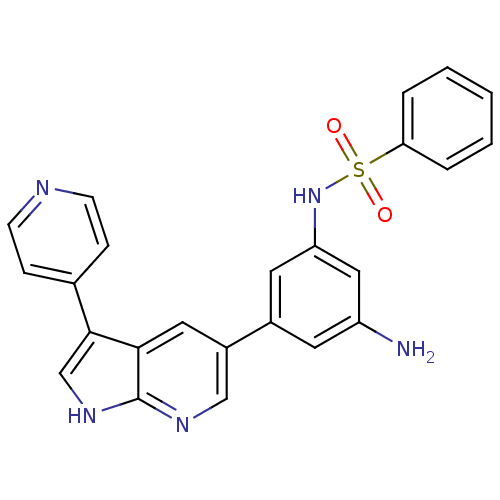
(CHEMBL2152285)Show SMILES Nc1cc(NS(=O)(=O)c2ccccc2)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H19N5O2S/c25-19-10-17(11-20(13-19)29-32(30,31)21-4-2-1-3-5-21)18-12-22-23(15-28-24(22)27-14-18)16-6-8-26-9-7-16/h1-15,29H,25H2,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391973
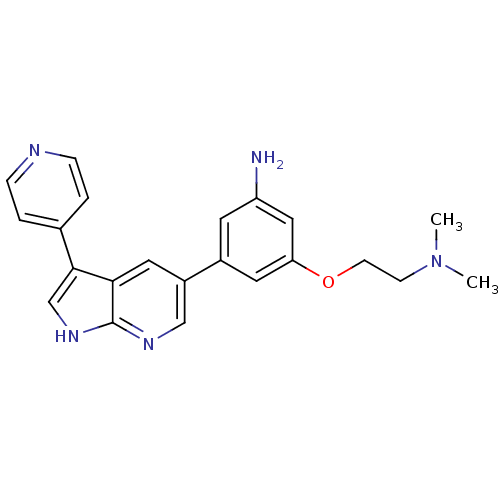
(CHEMBL2152282)Show SMILES CN(C)CCOc1cc(N)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C22H23N5O/c1-27(2)7-8-28-19-10-16(9-18(23)12-19)17-11-20-21(14-26-22(20)25-13-17)15-3-5-24-6-4-15/h3-6,9-14H,7-8,23H2,1-2H3,(H,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391971

(CHEMBL2152280)Show SMILES COc1cc(N)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C19H16N4O/c1-24-16-7-13(6-15(20)9-16)14-8-17-18(11-23-19(17)22-10-14)12-2-4-21-5-3-12/h2-11H,20H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391968
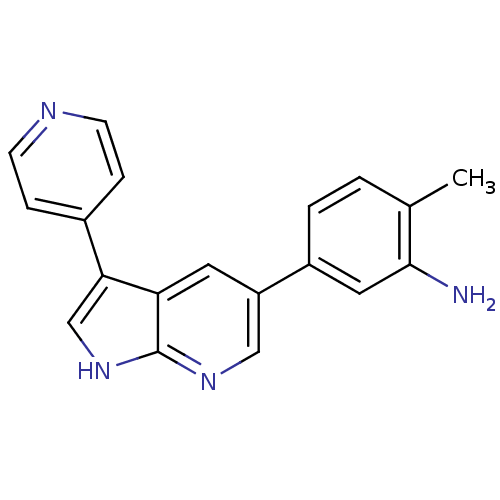
(CHEMBL2152277)Show InChI InChI=1S/C19H16N4/c1-12-2-3-14(9-18(12)20)15-8-16-17(11-23-19(16)22-10-15)13-4-6-21-7-5-13/h2-11H,20H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391963
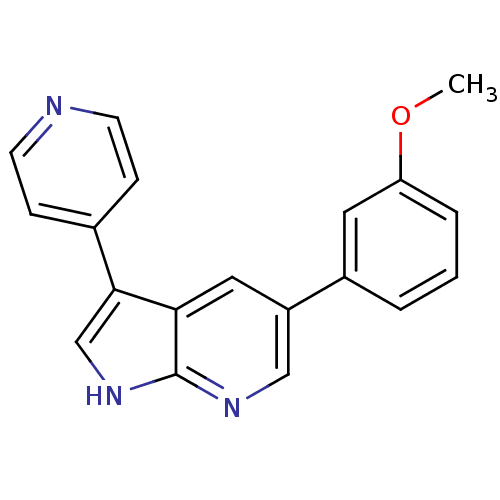
(CHEMBL2152272)Show InChI InChI=1S/C19H15N3O/c1-23-16-4-2-3-14(9-16)15-10-17-18(12-22-19(17)21-11-15)13-5-7-20-8-6-13/h2-12H,1H3,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391965
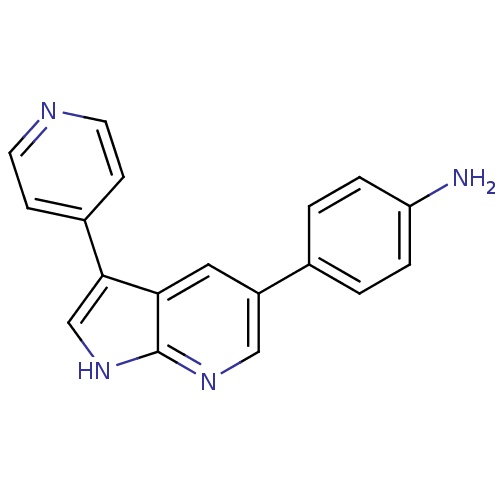
(CHEMBL2152274)Show InChI InChI=1S/C18H14N4/c19-15-3-1-12(2-4-15)14-9-16-17(11-22-18(16)21-10-14)13-5-7-20-8-6-13/h1-11H,19H2,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391962

(CHEMBL2152271)Show SMILES CN(C)c1cccc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C20H18N4/c1-24(2)17-5-3-4-15(10-17)16-11-18-19(13-23-20(18)22-12-16)14-6-8-21-9-7-14/h3-13H,1-2H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391961
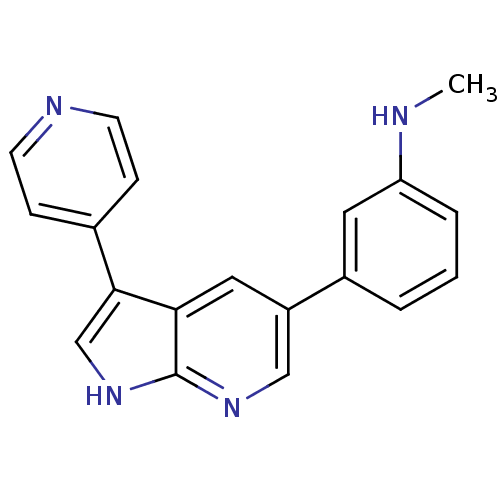
(CHEMBL2152270)Show InChI InChI=1S/C19H16N4/c1-20-16-4-2-3-14(9-16)15-10-17-18(12-23-19(17)22-11-15)13-5-7-21-8-6-13/h2-12,20H,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391966

(CHEMBL2152275)Show InChI InChI=1S/C19H16N4O/c1-24-18-3-2-13(9-17(18)20)14-8-15-16(11-23-19(15)22-10-14)12-4-6-21-7-5-12/h2-11H,20H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391975
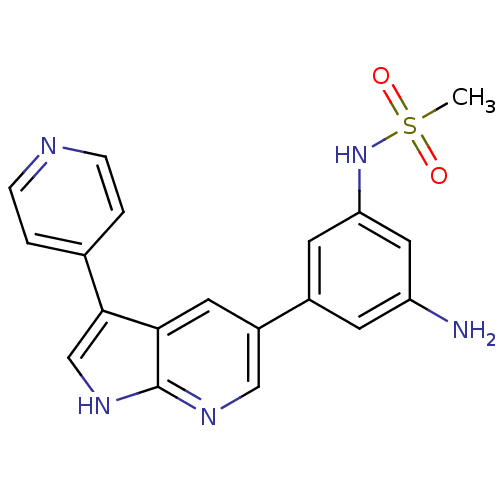
(CHEMBL2152284)Show SMILES CS(=O)(=O)Nc1cc(N)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C19H17N5O2S/c1-27(25,26)24-16-7-13(6-15(20)9-16)14-8-17-18(11-23-19(17)22-10-14)12-2-4-21-5-3-12/h2-11,24H,20H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391964
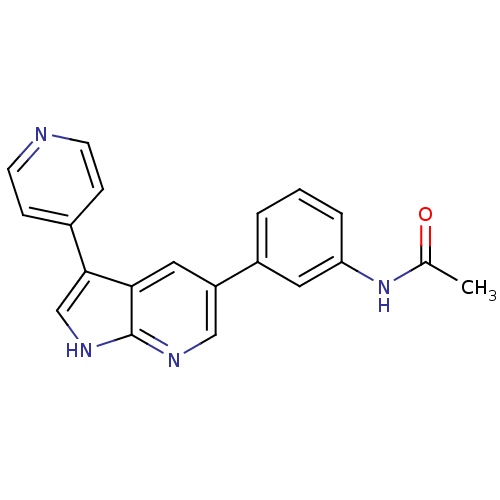
(CHEMBL2152273)Show SMILES CC(=O)Nc1cccc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C20H16N4O/c1-13(25)24-17-4-2-3-15(9-17)16-10-18-19(12-23-20(18)22-11-16)14-5-7-21-8-6-14/h2-12H,1H3,(H,22,23)(H,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50222222
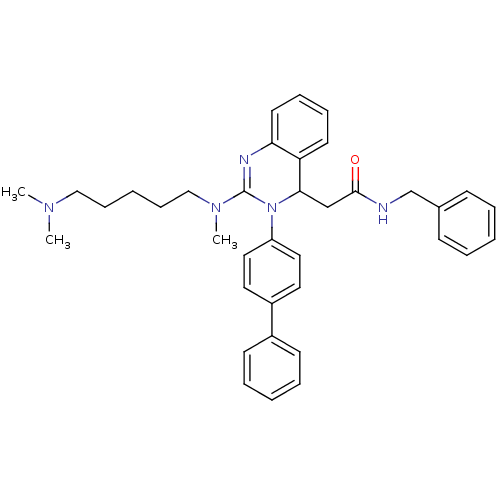
(CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...)Show SMILES CN(C)CCCCCN(C)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:10| Show InChI InChI=1S/C37H43N5O/c1-40(2)25-13-6-14-26-41(3)37-39-34-20-12-11-19-33(34)35(27-36(43)38-28-29-15-7-4-8-16-29)42(37)32-23-21-31(22-24-32)30-17-9-5-10-18-30/h4-5,7-12,15-24,35H,6,13-14,25-28H2,1-3H3,(H,38,43) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of T-type calcium channel alpha 1 G |
Bioorg Med Chem Lett 22: 1198-201 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.083
BindingDB Entry DOI: 10.7270/Q2GQ6Z59 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391955

(CHEMBL2152264)Show InChI InChI=1S/C18H14N4/c19-15-3-1-2-13(8-15)14-9-16-17(11-22-18(16)21-10-14)12-4-6-20-7-5-12/h1-11H,19H2,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391970
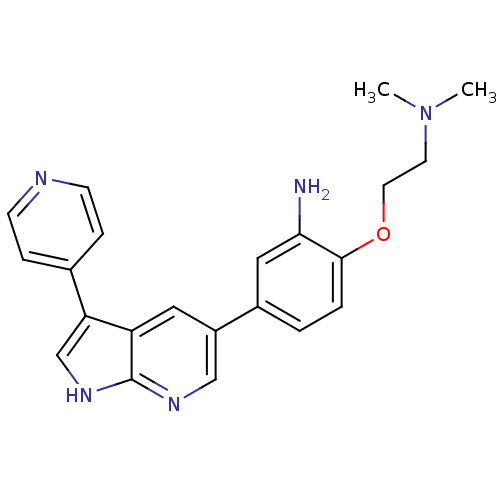
(CHEMBL2152279)Show SMILES CN(C)CCOc1ccc(cc1N)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C22H23N5O/c1-27(2)9-10-28-21-4-3-16(12-20(21)23)17-11-18-19(14-26-22(18)25-13-17)15-5-7-24-8-6-15/h3-8,11-14H,9-10,23H2,1-2H3,(H,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391967
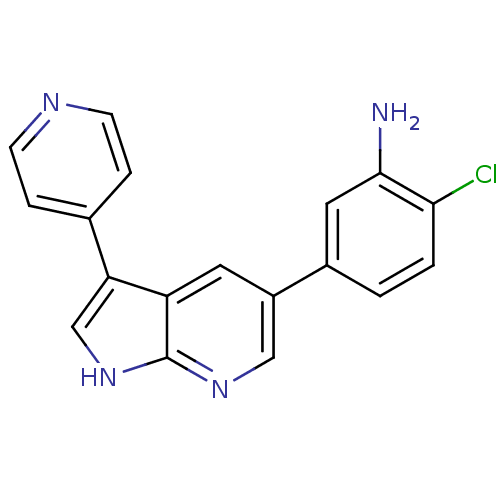
(CHEMBL2152276)Show InChI InChI=1S/C18H13ClN4/c19-16-2-1-12(8-17(16)20)13-7-14-15(10-23-18(14)22-9-13)11-3-5-21-6-4-11/h1-10H,20H2,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50495798
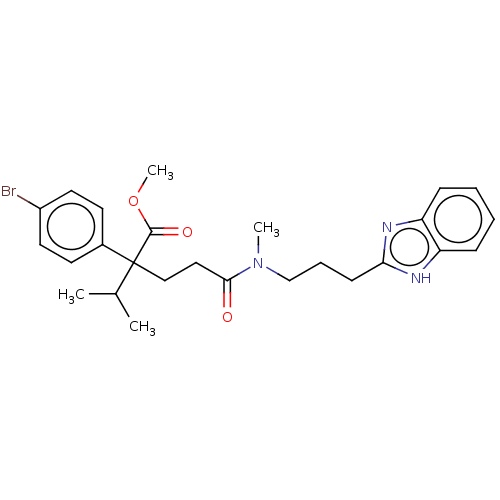
(CHEMBL3115194)Show SMILES COC(=O)C(CCC(=O)N(C)CCCc1nc2ccccc2[nH]1)(C(C)C)c1ccc(Br)cc1 Show InChI InChI=1S/C26H32BrN3O3/c1-18(2)26(25(32)33-4,19-11-13-20(27)14-12-19)16-15-24(31)30(3)17-7-10-23-28-21-8-5-6-9-22(21)29-23/h5-6,8-9,11-14,18H,7,10,15-17H2,1-4H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of T-type calcium channel subunit alpha-1G (unknown origin) expressed in HEK293 cells assessed as inactivation of channel current at holdi... |
Bioorg Med Chem Lett 24: 880-3 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.078
BindingDB Entry DOI: 10.7270/Q2C53PSG |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391972
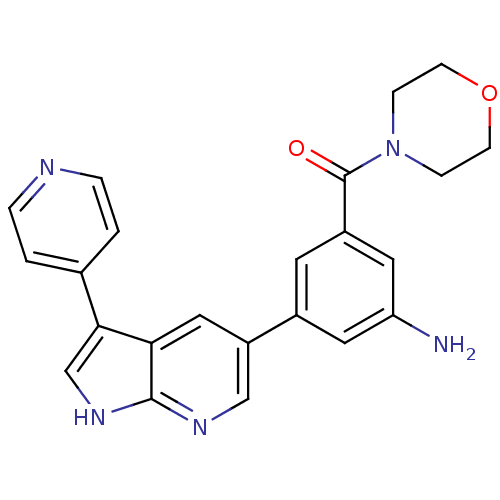
(CHEMBL2152281)Show SMILES Nc1cc(cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C23H21N5O2/c24-19-10-16(9-17(11-19)23(29)28-5-7-30-8-6-28)18-12-20-21(14-27-22(20)26-13-18)15-1-3-25-4-2-15/h1-4,9-14H,5-8,24H2,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391960
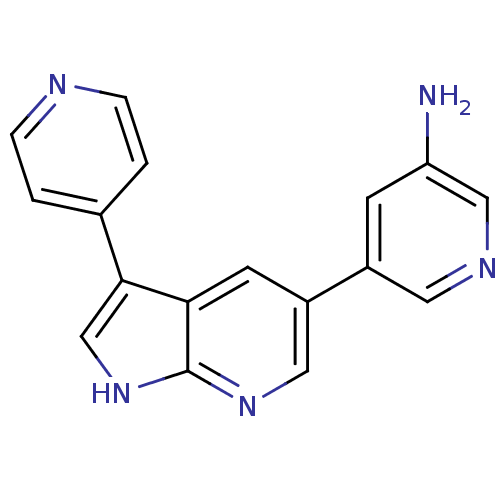
(CHEMBL2152269)Show InChI InChI=1S/C17H13N5/c18-14-5-12(7-20-9-14)13-6-15-16(10-22-17(15)21-8-13)11-1-3-19-4-2-11/h1-10H,18H2,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123843
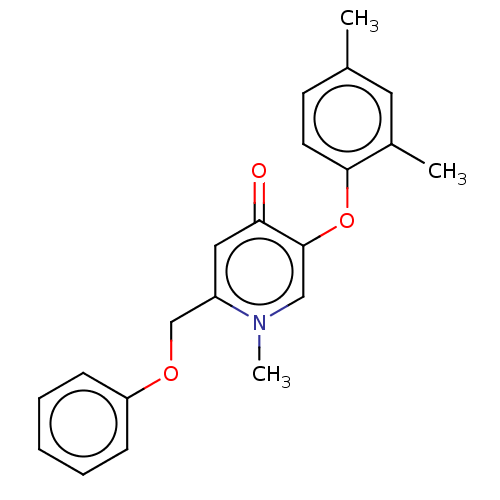
(CHEMBL3623427)Show InChI InChI=1S/C21H21NO3/c1-15-9-10-20(16(2)11-15)25-21-13-22(3)17(12-19(21)23)14-24-18-7-5-4-6-8-18/h4-13H,14H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391969

(CHEMBL2152278)Show SMILES Nc1cc(ccc1C(=O)N1CCOCC1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H21N5O2/c24-21-12-16(1-2-18(21)23(29)28-7-9-30-10-8-28)17-11-19-20(14-27-22(19)26-13-17)15-3-5-25-6-4-15/h1-6,11-14H,7-10,24H2,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50262239
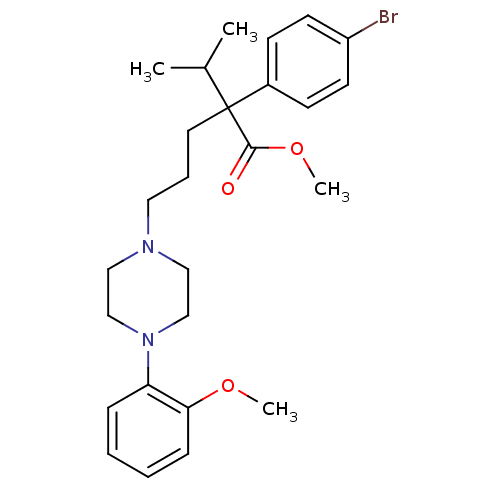
(CHEMBL513338 | methyl 2-(4-bromophenyl)-2-isopropy...)Show SMILES COC(=O)C(CCCN1CCN(CC1)c1ccccc1OC)(C(C)C)c1ccc(Br)cc1 Show InChI InChI=1S/C26H35BrN2O3/c1-20(2)26(25(30)32-4,21-10-12-22(27)13-11-21)14-7-15-28-16-18-29(19-17-28)23-8-5-6-9-24(23)31-3/h5-6,8-13,20H,7,14-19H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of T-type calcium channel subunit alpha-1G (unknown origin) expressed in HEK293 cells assessed as inactivation of channel current at holdi... |
Bioorg Med Chem Lett 24: 880-3 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.078
BindingDB Entry DOI: 10.7270/Q2C53PSG |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50459685
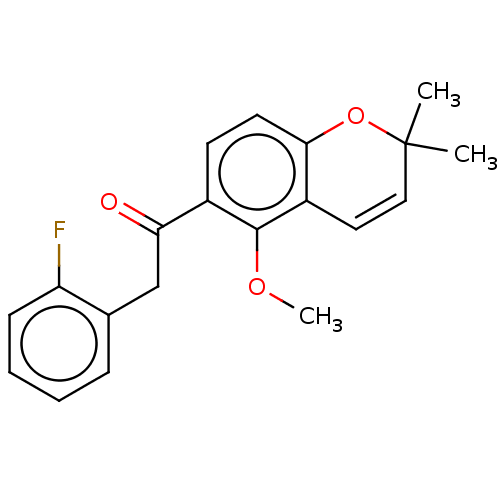
(CHEMBL4209785)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C(=O)Cc1ccccc1F |c:11| Show InChI InChI=1S/C20H19FO3/c1-20(2)11-10-15-18(24-20)9-8-14(19(15)23-3)17(22)12-13-6-4-5-7-16(13)21/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human HRE-A549 cells pre-incubated for 1 hr before exposure to hypoxia for 24 hrs by HRE-luciferase reporter gene assay |
J Med Chem 61: 9266-9286 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00971
BindingDB Entry DOI: 10.7270/Q269766D |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123848

(CHEMBL3623422)Show InChI InChI=1S/C20H18ClNO3/c1-14-10-15(21)8-9-19(14)25-20-12-22(2)16(11-18(20)23)13-24-17-6-4-3-5-7-17/h3-12H,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50391951
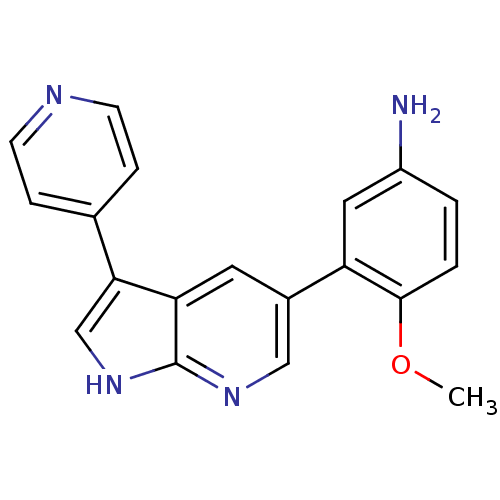
(CHEMBL2152290)Show InChI InChI=1S/C19H16N4O/c1-24-18-3-2-14(20)9-15(18)13-8-16-17(11-23-19(16)22-10-13)12-4-6-21-7-5-12/h2-11H,20H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA assessed as [gamma33P]ATP incorporation into substrate |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123853
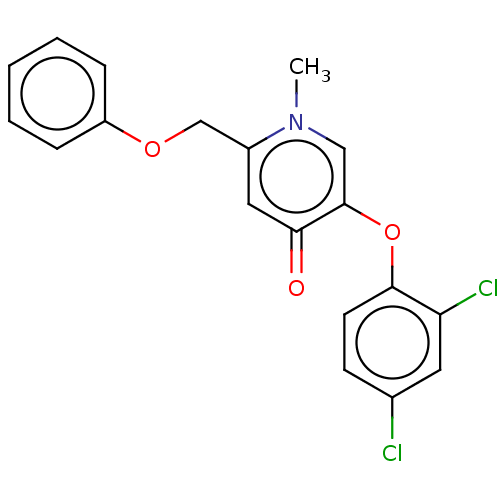
(CHEMBL3623417)Show InChI InChI=1S/C19H15Cl2NO3/c1-22-11-19(25-18-8-7-13(20)9-16(18)21)17(23)10-14(22)12-24-15-5-3-2-4-6-15/h2-11H,12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50391953

(CHEMBL2152288)Show SMILES Nc1cc(NS(=O)(=O)c2cccc(c2)C(F)(F)F)cc(c1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H18F3N5O2S/c26-25(27,28)18-2-1-3-21(11-18)36(34,35)33-20-9-16(8-19(29)12-20)17-10-22-23(14-32-24(22)31-13-17)15-4-6-30-7-5-15/h1-14,33H,29H2,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology (KAIST)
Curated by ChEMBL
| Assay Description
Inhibition of KIT by high throughput binding assay |
J Med Chem 55: 5337-49 (2012)
Article DOI: 10.1021/jm3002982
BindingDB Entry DOI: 10.7270/Q2JM2BQ3 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50390272

(CHEMBL2070415)Show SMILES CN1CCN(CCCCCOc2ccc(cc2)N2C(=S)S\C(=C/c3ccc(Oc4ccc(cc4)C(N)=O)cc3)C2=O)CC1 Show InChI InChI=1S/C33H36N4O4S2/c1-35-18-20-36(21-19-35)17-3-2-4-22-40-27-15-9-26(10-16-27)37-32(39)30(43-33(37)42)23-24-5-11-28(12-6-24)41-29-13-7-25(8-14-29)31(34)38/h5-16,23H,2-4,17-22H2,1H3,(H2,34,38)/b30-23- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta using 5FAM-GRHDSGLDSMK-NH2 as substrate incubated for 10 mins prior to substrate addition by IMAP-TR-FRET assay |
Bioorg Med Chem Lett 22: 5668-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.088
BindingDB Entry DOI: 10.7270/Q2NZ88QX |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149806
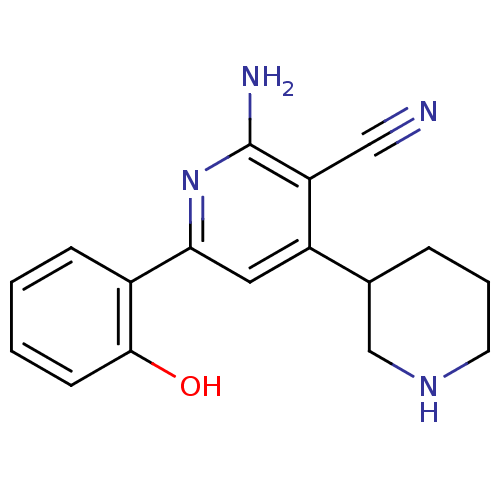
(2''-Amino-6''-(2-hydroxy-phenyl)-1,2,3,4,5,6-hexah...)Show InChI InChI=1S/C17H18N4O/c18-9-14-13(11-4-3-7-20-10-11)8-15(21-17(14)19)12-5-1-2-6-16(12)22/h1-2,5-6,8,11,20,22H,3-4,7,10H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta using 5FAM-GRHDSGLDSMK-NH2 as substrate incubated for 10 mins prior to substrate addition by IMAP-TR-FRET assay |
Bioorg Med Chem Lett 22: 5668-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.088
BindingDB Entry DOI: 10.7270/Q2NZ88QX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50262085

(CHEMBL468397 | methyl 5-((3-(1H-benzo[d]imidazol-2...)Show SMILES COC(=O)C(CCCN(C)CCCc1nc2ccccc2[nH]1)(C(C)C)c1ccc(Br)cc1 Show InChI InChI=1S/C26H34BrN3O2/c1-19(2)26(25(31)32-4,20-12-14-21(27)15-13-20)16-8-18-30(3)17-7-11-24-28-22-9-5-6-10-23(22)29-24/h5-6,9-10,12-15,19H,7-8,11,16-18H2,1-4H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by whole-cell patch clamp method |
Bioorg Med Chem Lett 24: 880-3 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.078
BindingDB Entry DOI: 10.7270/Q2C53PSG |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123838
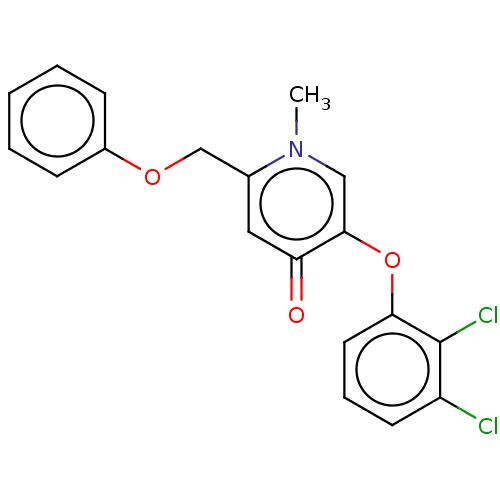
(CHEMBL3623432)Show InChI InChI=1S/C19H15Cl2NO3/c1-22-11-18(25-17-9-5-8-15(20)19(17)21)16(23)10-13(22)12-24-14-6-3-2-4-7-14/h2-11H,12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50390261

(CHEMBL2070414)Show SMILES CN1CCN(CCCCOc2ccc(cc2)N2C(=S)S\C(=C/c3ccc(Oc4ccc(cc4)C(N)=O)cc3)C2=O)CC1 Show InChI InChI=1S/C32H34N4O4S2/c1-34-17-19-35(20-18-34)16-2-3-21-39-26-14-8-25(9-15-26)36-31(38)29(42-32(36)41)22-23-4-10-27(11-5-23)40-28-12-6-24(7-13-28)30(33)37/h4-15,22H,2-3,16-21H2,1H3,(H2,33,37)/b29-22- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta using 5FAM-GRHDSGLDSMK-NH2 as substrate incubated for 10 mins prior to substrate addition by IMAP-TR-FRET assay |
Bioorg Med Chem Lett 22: 5668-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.088
BindingDB Entry DOI: 10.7270/Q2NZ88QX |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50390260

(CHEMBL2070413)Show SMILES CN1CCN(CCCOc2ccc(cc2)N2C(=S)S\C(=C/c3ccc(Oc4ccc(cc4)C(N)=O)cc3)C2=O)CC1 Show InChI InChI=1S/C31H32N4O4S2/c1-33-16-18-34(19-17-33)15-2-20-38-25-13-7-24(8-14-25)35-30(37)28(41-31(35)40)21-22-3-9-26(10-4-22)39-27-11-5-23(6-12-27)29(32)36/h3-14,21H,2,15-20H2,1H3,(H2,32,36)/b28-21- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta (unknown origin) by TR-FRET assay |
Bioorg Med Chem Lett 26: 1120-3 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.065
BindingDB Entry DOI: 10.7270/Q2F76FD3 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50390260

(CHEMBL2070413)Show SMILES CN1CCN(CCCOc2ccc(cc2)N2C(=S)S\C(=C/c3ccc(Oc4ccc(cc4)C(N)=O)cc3)C2=O)CC1 Show InChI InChI=1S/C31H32N4O4S2/c1-33-16-18-34(19-17-33)15-2-20-38-25-13-7-24(8-14-25)35-30(37)28(41-31(35)40)21-22-3-9-26(10-4-22)39-27-11-5-23(6-12-27)29(32)36/h3-14,21H,2,15-20H2,1H3,(H2,32,36)/b28-21- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta using 5FAM-GRHDSGLDSMK-NH2 as substrate incubated for 10 mins prior to substrate addition by IMAP-TR-FRET assay |
Bioorg Med Chem Lett 22: 5668-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.088
BindingDB Entry DOI: 10.7270/Q2NZ88QX |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50459697
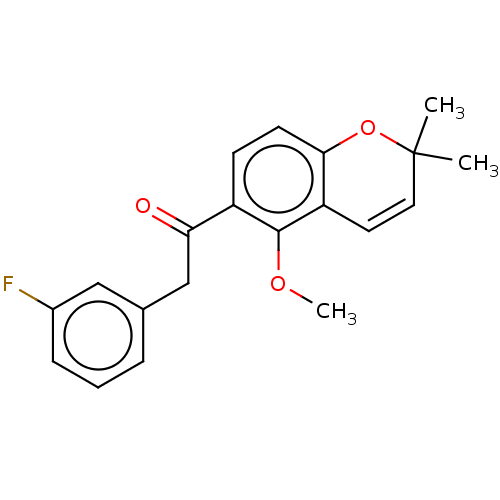
(CHEMBL4214693)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C(=O)Cc1cccc(F)c1 |c:11| Show InChI InChI=1S/C20H19FO3/c1-20(2)10-9-16-18(24-20)8-7-15(19(16)23-3)17(22)12-13-5-4-6-14(21)11-13/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human HRE-A549 cells pre-incubated for 1 hr before exposure to hypoxia for 24 hrs by HRE-luciferase reporter gene assay |
J Med Chem 61: 9266-9286 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00971
BindingDB Entry DOI: 10.7270/Q269766D |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123827
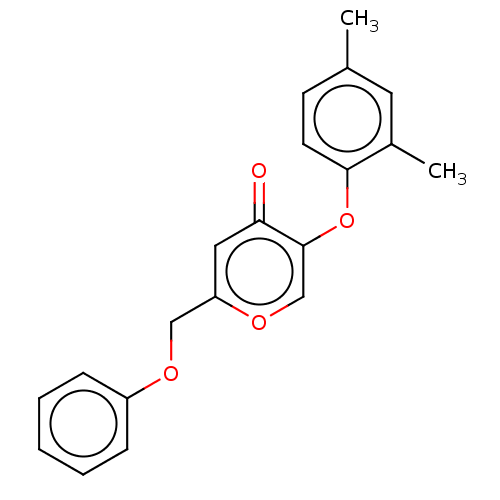
(CHEMBL3623392)Show InChI InChI=1S/C20H18O4/c1-14-8-9-19(15(2)10-14)24-20-13-23-17(11-18(20)21)12-22-16-6-4-3-5-7-16/h3-11,13H,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123849
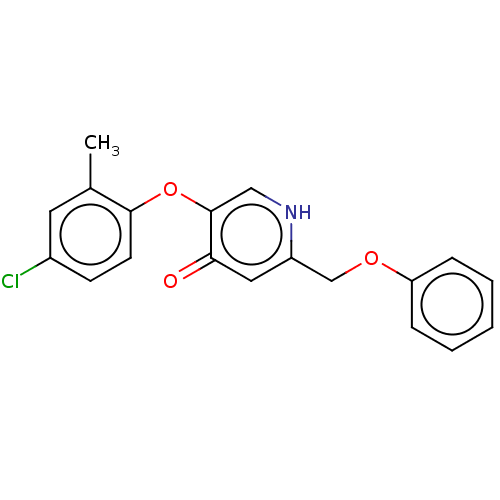
(CHEMBL3623421)Show InChI InChI=1S/C19H16ClNO3/c1-13-9-14(20)7-8-18(13)24-19-11-21-15(10-17(19)22)12-23-16-5-3-2-4-6-16/h2-11H,12H2,1H3,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123828
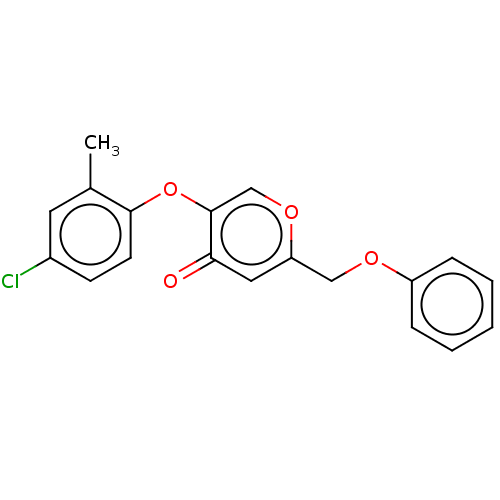
(CHEMBL3623391)Show InChI InChI=1S/C19H15ClO4/c1-13-9-14(20)7-8-18(13)24-19-12-23-16(10-17(19)21)11-22-15-5-3-2-4-6-15/h2-10,12H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123847
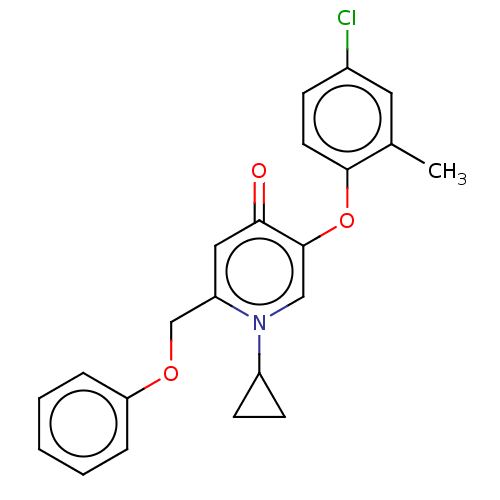
(CHEMBL3623423)Show SMILES Cc1cc(Cl)ccc1Oc1cn(C2CC2)c(COc2ccccc2)cc1=O Show InChI InChI=1S/C22H20ClNO3/c1-15-11-16(23)7-10-21(15)27-22-13-24(17-8-9-17)18(12-20(22)25)14-26-19-5-3-2-4-6-19/h2-7,10-13,17H,8-9,14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50123852
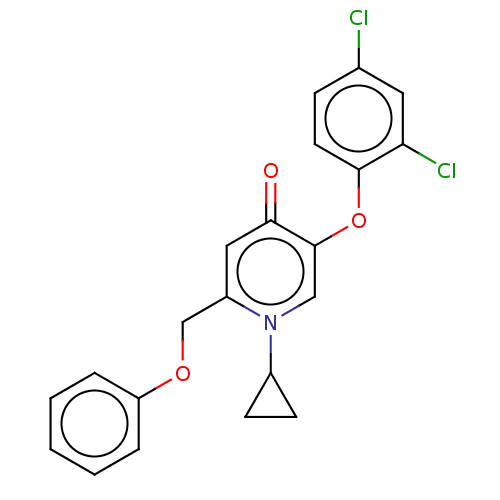
(CHEMBL3623418)Show SMILES Clc1ccc(Oc2cn(C3CC3)c(COc3ccccc3)cc2=O)c(Cl)c1 Show InChI InChI=1S/C21H17Cl2NO3/c22-14-6-9-20(18(23)10-14)27-21-12-24(15-7-8-15)16(11-19(21)25)13-26-17-4-2-1-3-5-17/h1-6,9-12,15H,7-8,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI assessed as reduction in inhibition of reduction of trans-2-octenoyl N-acetylcysteamine substrate by spectro... |
Bioorg Med Chem Lett 25: 4481-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.077
BindingDB Entry DOI: 10.7270/Q2154JV0 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Mus musculus) | BDBM50146936
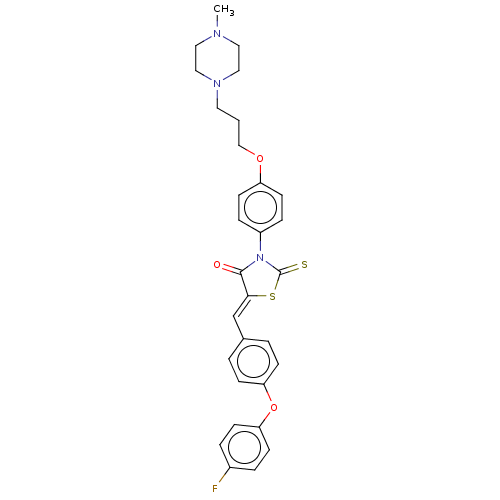
(CHEMBL3765499)Show SMILES CN1CCN(CCCOc2ccc(cc2)N2C(=S)S\C(=C/c3ccc(Oc4ccc(F)cc4)cc3)C2=O)CC1 Show InChI InChI=1S/C30H30FN3O3S2/c1-32-16-18-33(19-17-32)15-2-20-36-25-13-7-24(8-14-25)34-29(35)28(39-30(34)38)21-22-3-9-26(10-4-22)37-27-11-5-23(31)6-12-27/h3-14,21H,2,15-20H2,1H3/b28-21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Catholic University of Korea
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta in primary type-2 collagen immunized mouse splenocytes assessed as inhibition of TNFalpha production at 10 uM after 72 hrs by E... |
Bioorg Med Chem Lett 26: 1120-3 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.065
BindingDB Entry DOI: 10.7270/Q2F76FD3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50459699
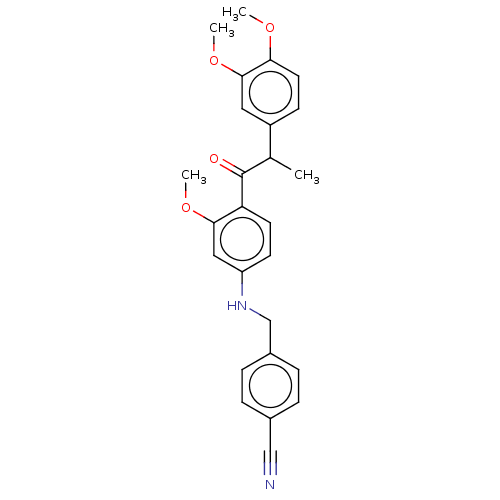
(CHEMBL4215206)Show SMILES COc1ccc(cc1OC)C(C)C(=O)c1ccc(NCc2ccc(cc2)C#N)cc1OC Show InChI InChI=1S/C26H26N2O4/c1-17(20-9-12-23(30-2)25(13-20)32-4)26(29)22-11-10-21(14-24(22)31-3)28-16-19-7-5-18(15-27)6-8-19/h5-14,17,28H,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human HRE-A549 cells pre-incubated for 1 hr before exposure to hypoxia for 24 hrs by HRE-luciferase reporter gene assay |
J Med Chem 61: 9266-9286 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00971
BindingDB Entry DOI: 10.7270/Q269766D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data