

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133473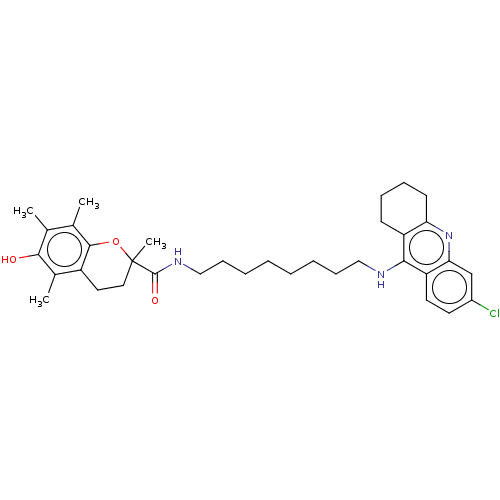 (CHEMBL3632994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550232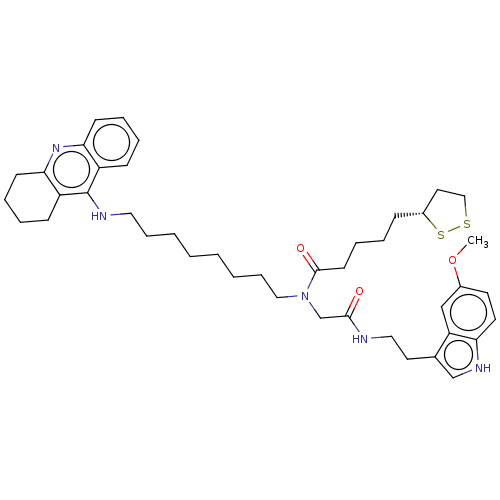 (CHEMBL4762083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550236 (CHEMBL4759893) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550234 (CHEMBL4784757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550235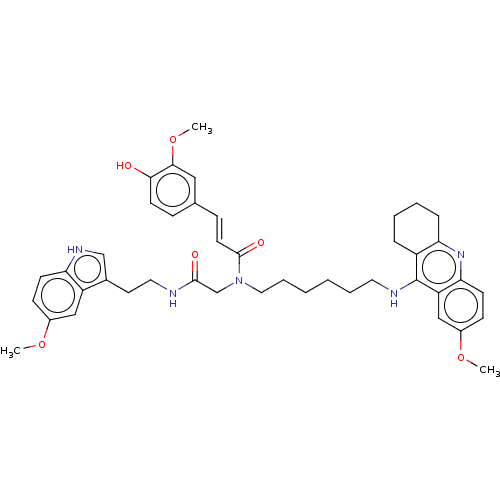 (CHEMBL4790426) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550232 (CHEMBL4762083) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550231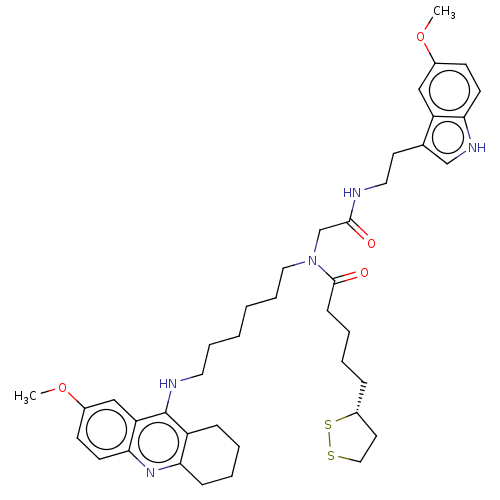 (CHEMBL4752488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550230 (CHEMBL4761802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550230 (CHEMBL4761802) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550229 (CHEMBL4755679) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550234 (CHEMBL4784757) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550233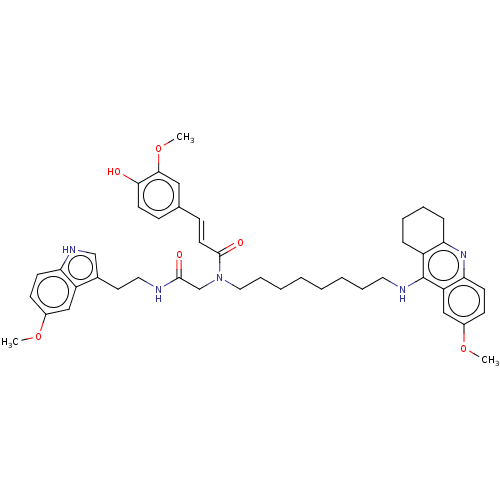 (CHEMBL4762095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550236 (CHEMBL4759893) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550229 (CHEMBL4755679) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550231 (CHEMBL4752488) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550235 (CHEMBL4790426) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133449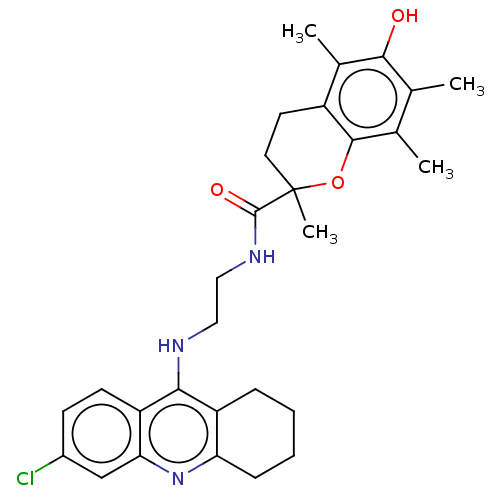 (CHEMBL3632988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550236 (CHEMBL4759893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550233 (CHEMBL4762095) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50550236 (CHEMBL4759893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's metho... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073117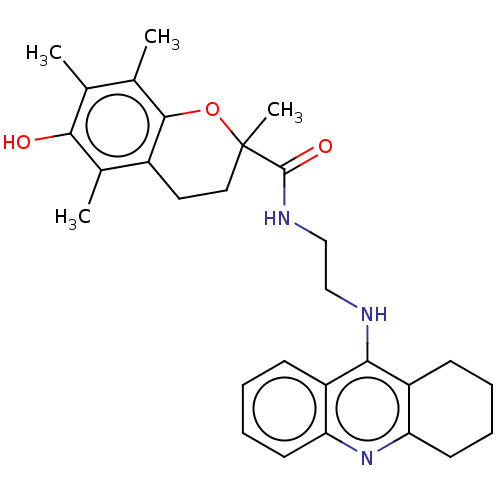 (CHEMBL3410951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133421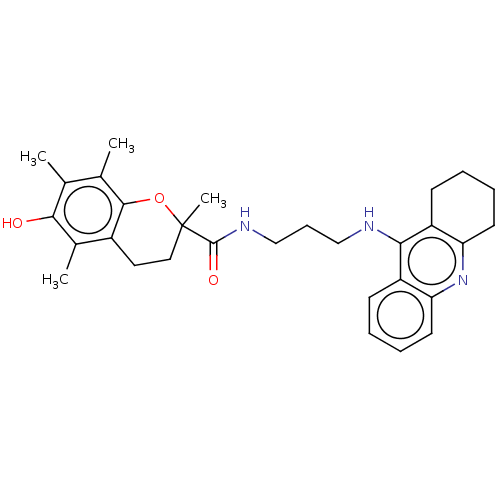 (CHEMBL3632987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133472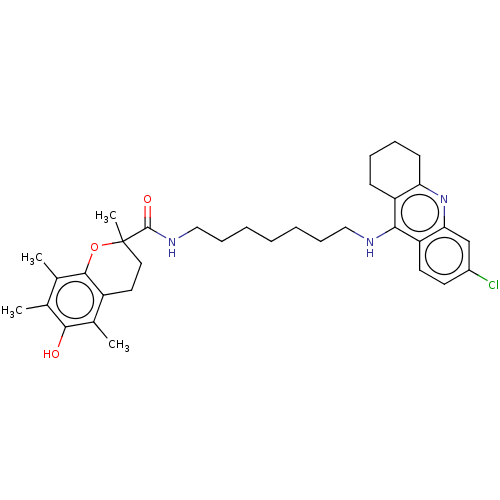 (CHEMBL3632993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133473 (CHEMBL3632994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073116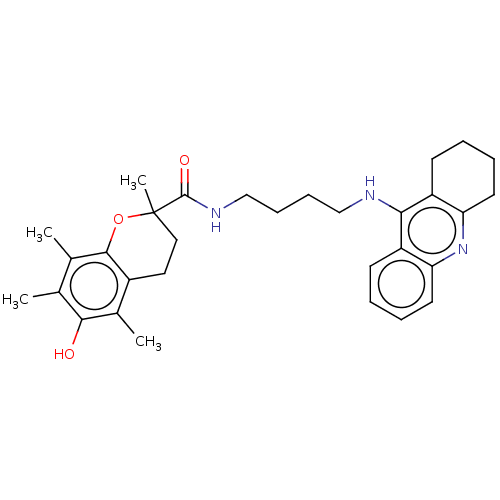 (CHEMBL3410952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133450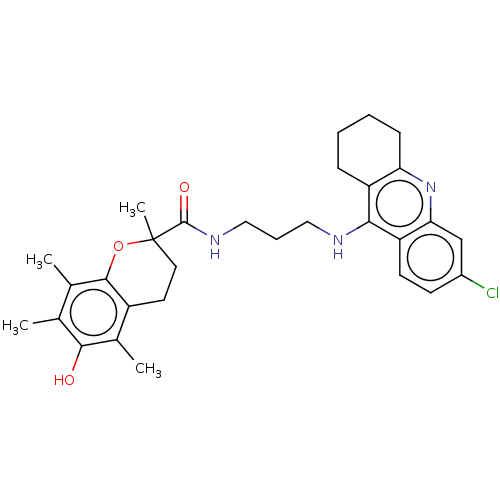 (CHEMBL3632989) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133469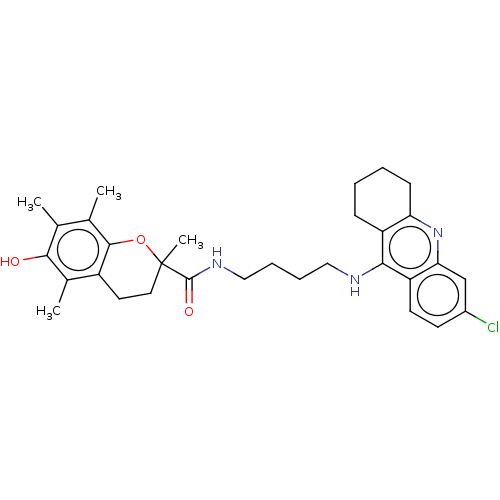 (CHEMBL3632990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133471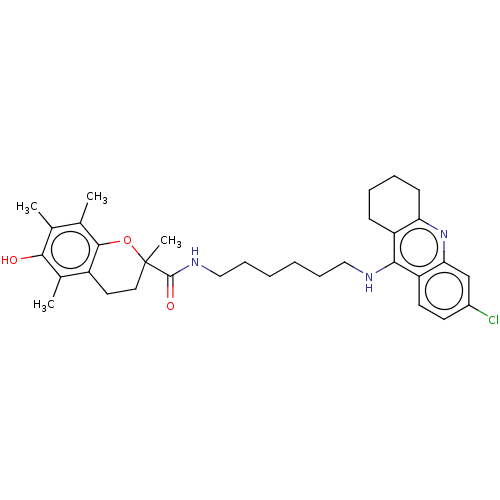 (CHEMBL3632992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133470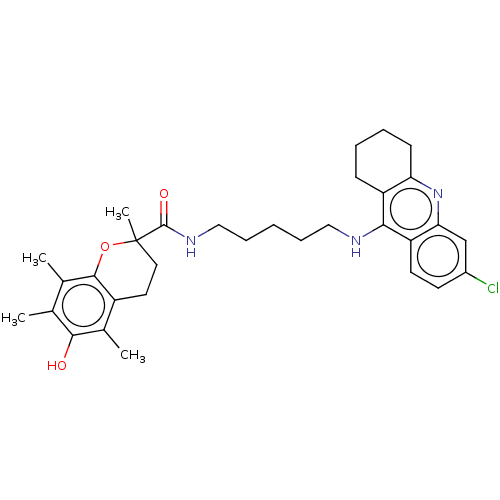 (CHEMBL3632991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133470 (CHEMBL3632991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073113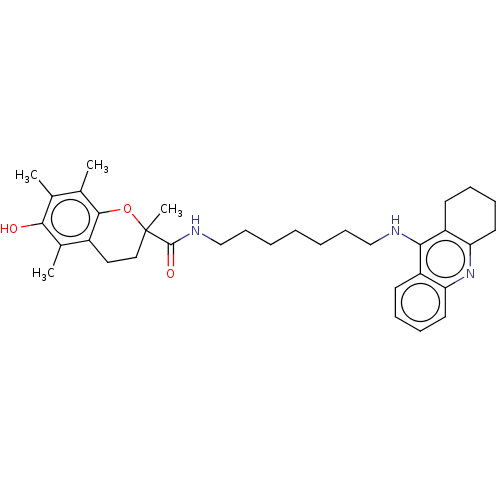 (CHEMBL3410955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133469 (CHEMBL3632990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073112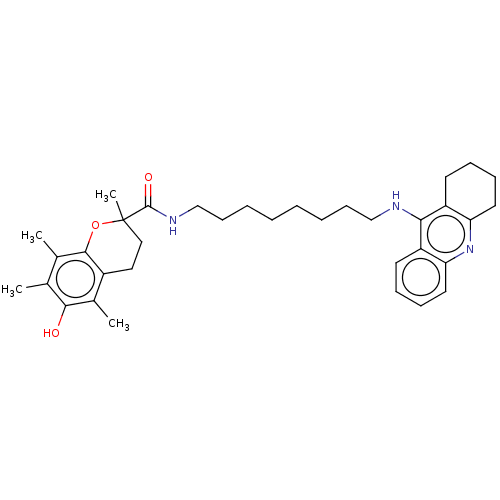 (CHEMBL3410956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50550232 (CHEMBL4762083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's metho... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133449 (CHEMBL3632988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550232 (CHEMBL4762083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550234 (CHEMBL4784757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133421 (CHEMBL3632987) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133412 (CHEMBL3632626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550233 (CHEMBL4762095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550235 (CHEMBL4790426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50073114 (CHEMBL3410954) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133413 (CHEMBL3632848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 92 total ) | Next | Last >> |