

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3570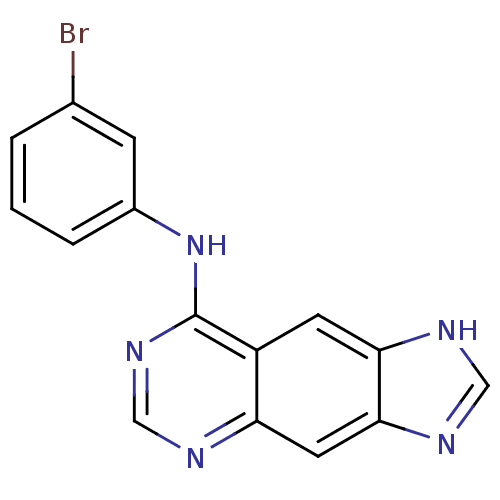 (8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3603 (4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3770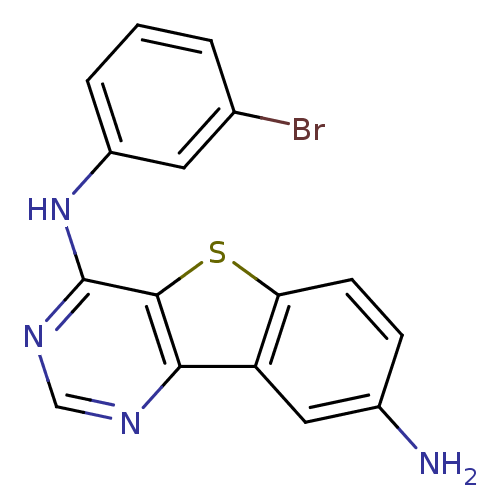 (6-N-(3-bromophenyl)-8-thia-3,5-diazatricyclo[7.4.0...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583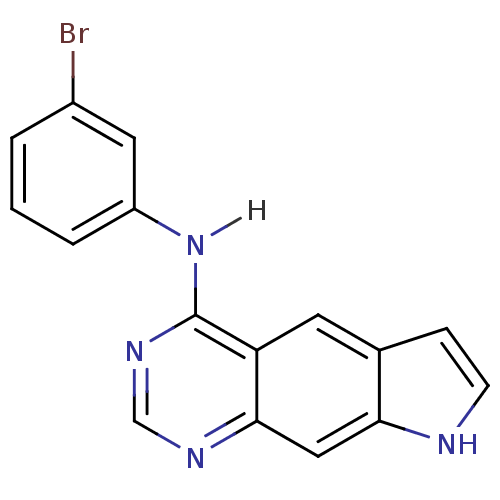 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3582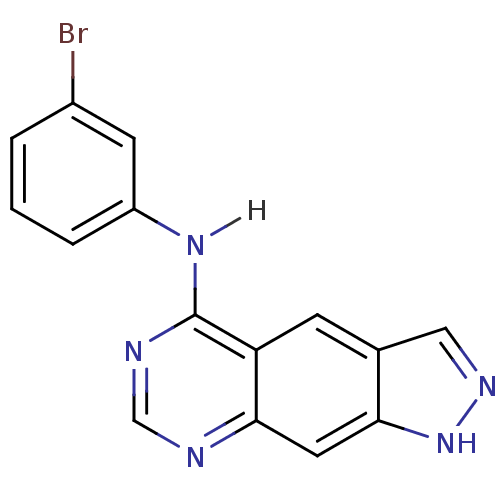 (5-[(3-Bromophenyl)amino]-1H-pyrazolo[4,3-g]quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3772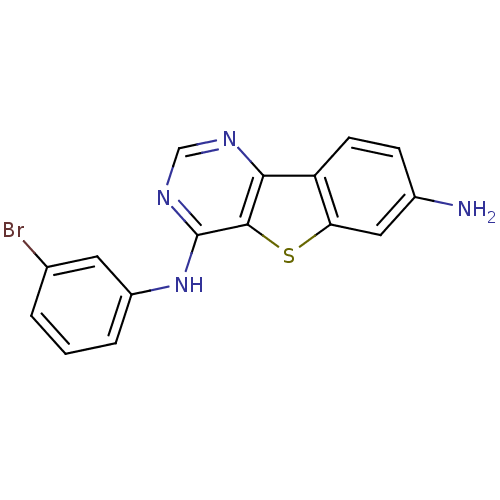 (6-N-(3-bromophenyl)-8-thia-3,5-diazatricyclo[7.4.0...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3773 (6-N-(3-bromophenyl)-12-fluoro-8-thia-3,5-diazatric...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3733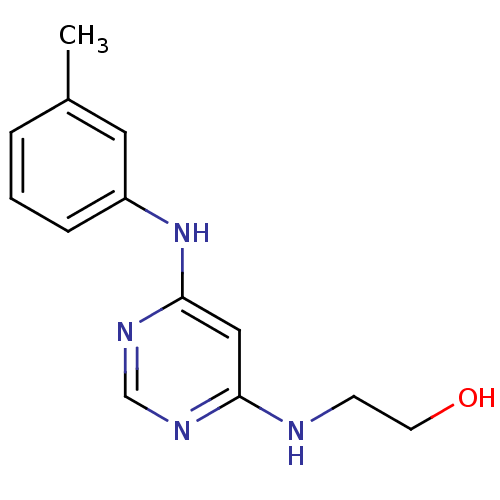 (2-({6-[(3-methylphenyl)amino]pyrimidin-4-yl}amino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3776 (4-(3-Bromoanilino)-8-methylaminobenzo[b]thieno[3,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3758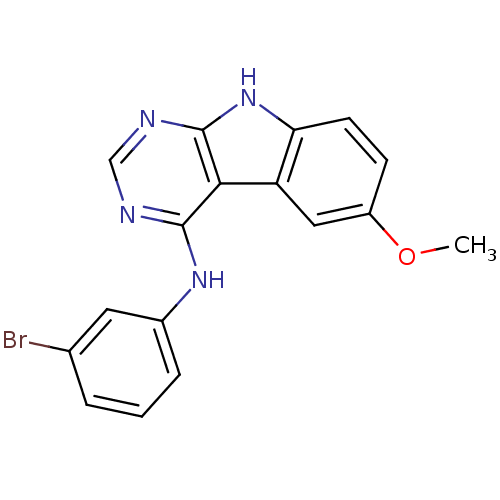 (N-(3-Bromophenyl)-6-methoxy-1H-pyrimido[4,5-b]indo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3584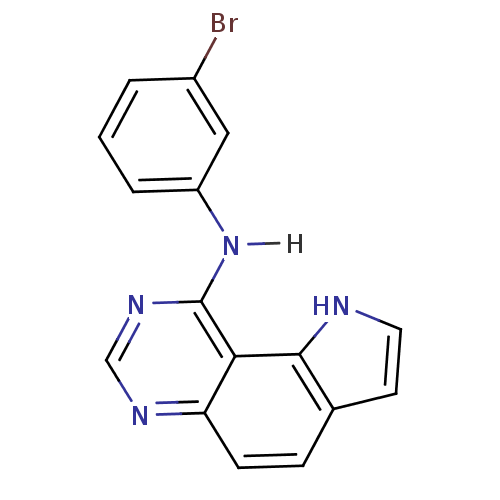 (9-[(3-Bromophenyl)amino]-1H-pyrrolo[2,3-f]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3762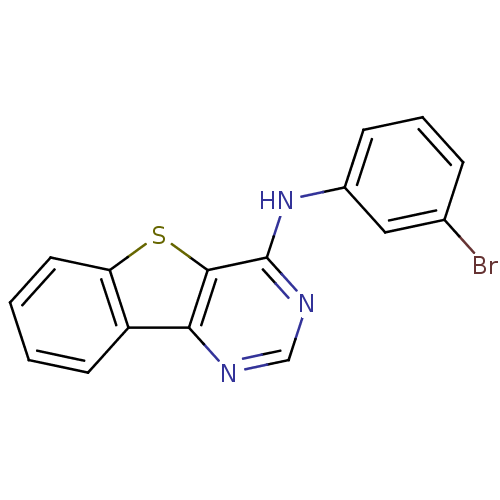 (Benzothieno[3,2-d]pyrimidine deriv. 53 | N-(3-brom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3768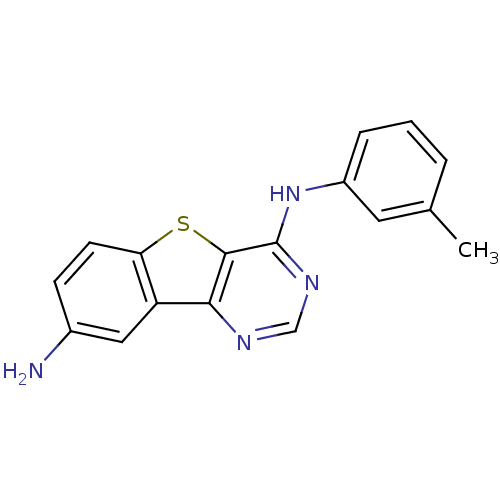 (6-N-(3-methylphenyl)-8-thia-3,5-diazatricyclo[7.4....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3780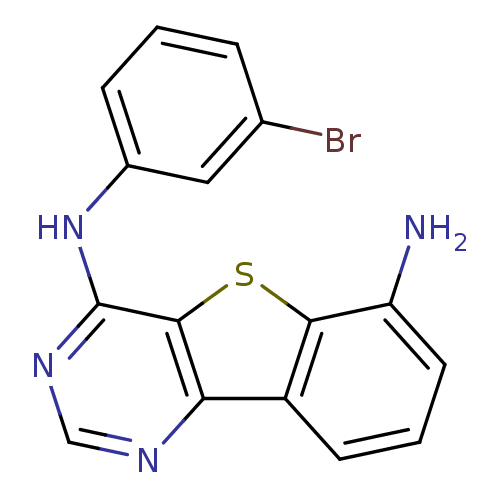 (6-N-(3-bromophenyl)-8-thia-3,5-diazatricyclo[7.4.0...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50044072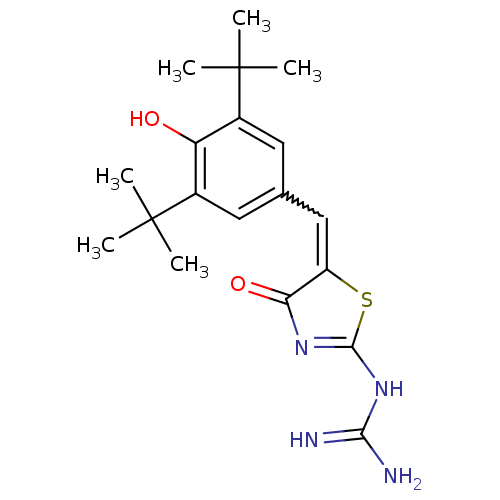 (CHEMBL16434 | N-[5-(3,5-Di-tert-butyl-4-hydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 against ovine Prostaglandin G/H synthase 1 | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3774 (6-N-(3-bromophenyl)-11-N-ethyl-12-fluoro-8-thia-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 1 of human platelet rich plasma | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 against Prostaglandin G/H synthase 1 from human platelet rich plasma | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50046902 (2,6-Di-tert-butyl-4-(5-methylsulfanyl-[1,3,4]thiad...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3767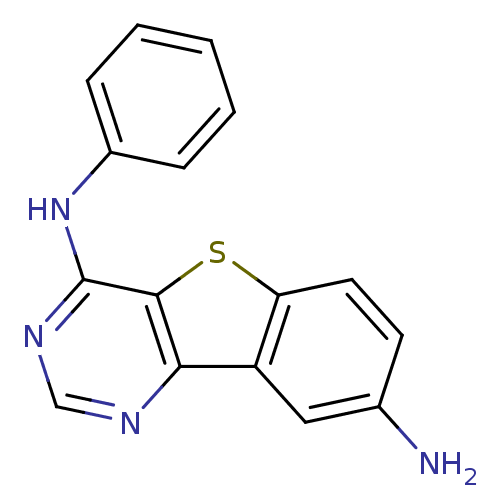 (6-N-phenyl-8-thia-3,5-diazatricyclo[7.4.0.0^{2,7}]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3764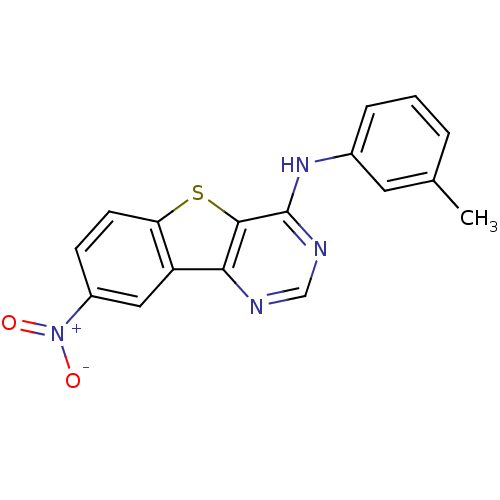 (Benzothieno[3,2-d]pyrimidine deriv. 55 | N-(3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3735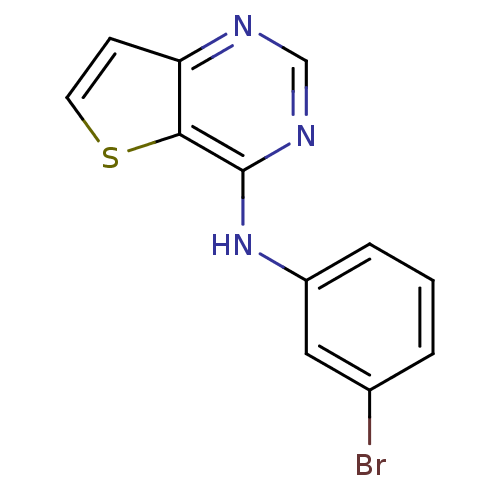 (Anilinopyrimidine deriv. 3 | N-(3-bromophenyl)thie...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50075529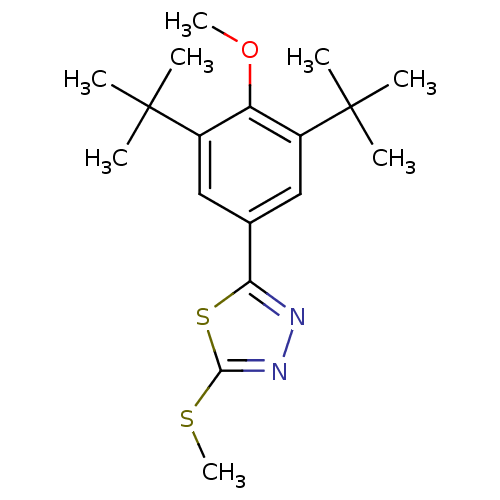 (2-(3,5-Di-tert-butyl-4-methoxy-phenyl)-5-methylsul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 against ovine Prostaglandin G/H synthase 1 | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against ovine Prostaglandin G/H synthase 1 | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3766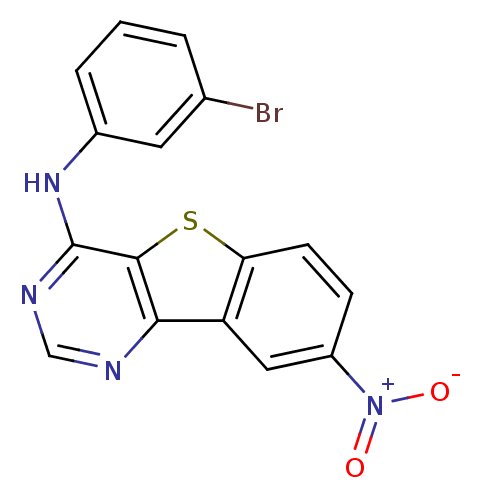 (Benzothieno[3,2-d]pyrimidine deriv. 57 | N-(3-brom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3778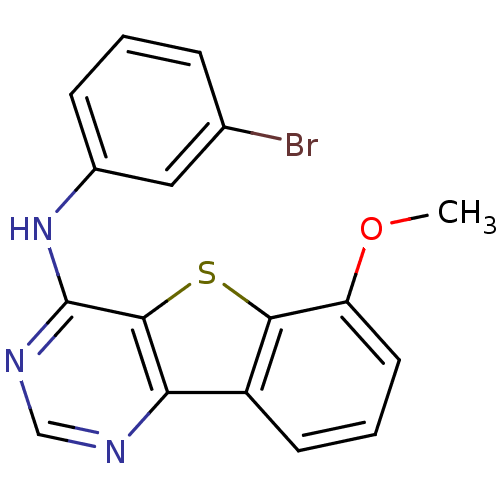 (Benzothieno[3,2-d]pyrimidine deriv. 69 | N-(3-brom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50075548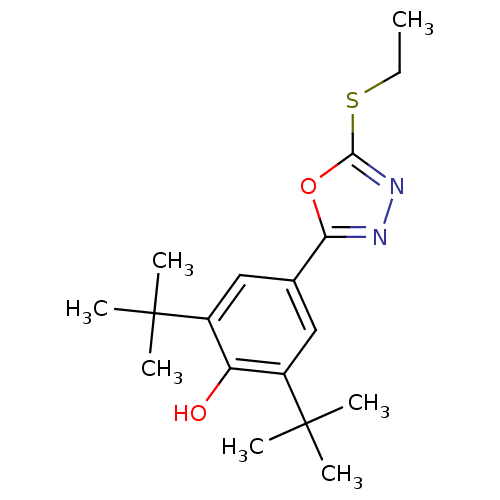 (2,6-Di-tert-butyl-4-(5-ethylsulfanyl-[1,3,4]oxadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50075541 (4,6-Di-tert-butyl-2-(5-ethylsulfanyl-[1,3,4]thiadi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50075551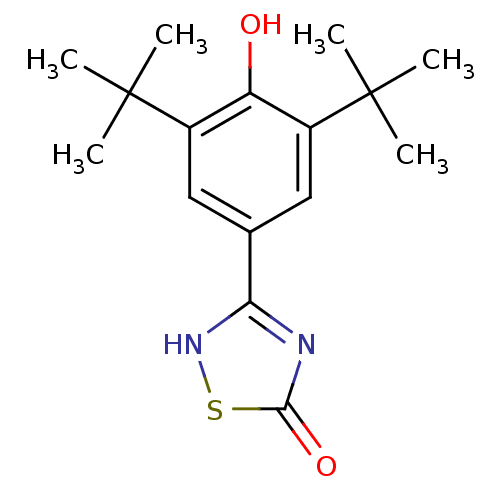 (3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3777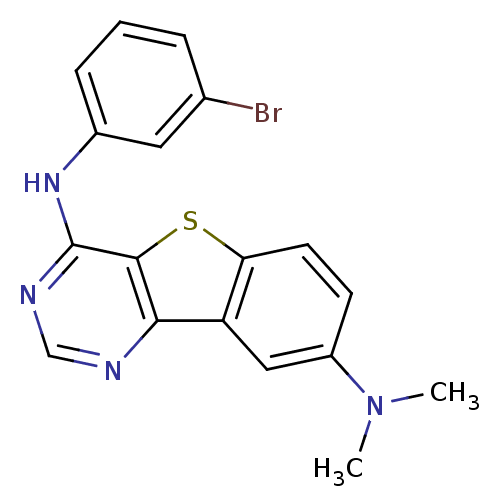 (4-(3-Bromoanilino)-8-dimethylaminobenzo[b]thieno[3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3781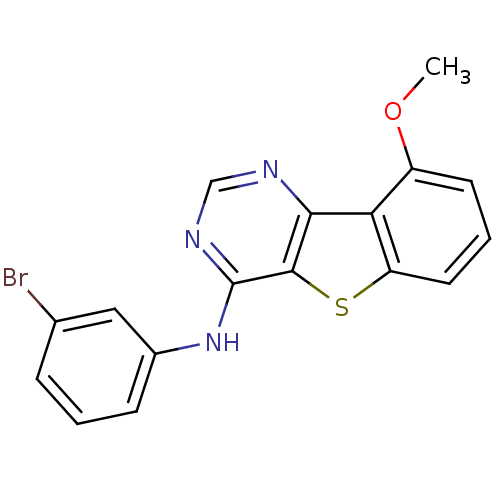 (Benzothieno[3,2-d]pyrimidine deriv. 72 | N-(3-brom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3263 (4-Anilino quinazoline deriv. 14 | CHEMBL329672 | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50075539 (3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50075519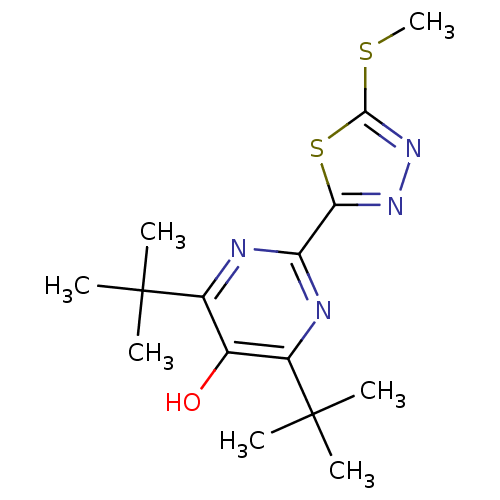 (4,6-Di-tert-butyl-2-(5-methylsulfanyl-[1,3,4]thiad...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50044068 (CHEMBL15904 | N-[5-(3,5-Di-tert-butyl-4-hydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 against ovine Prostaglandin G/H synthase 1 | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3577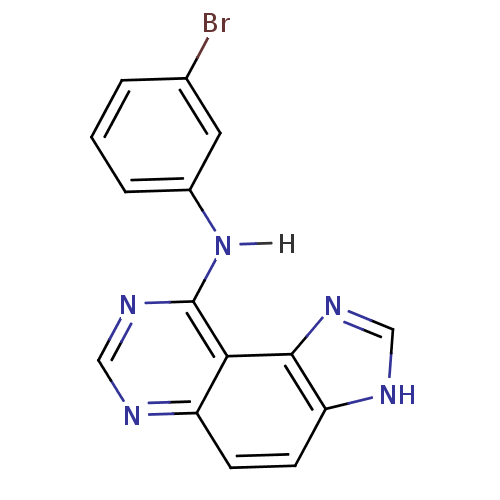 (9-[(3-Bromophenyl)amino]-1H-imidazo[4,5-f]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3753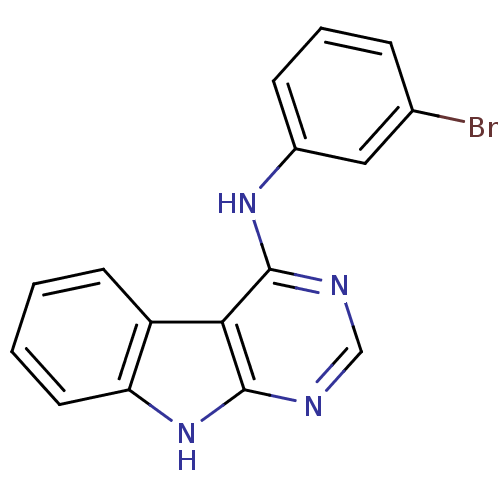 (N-(3-bromophenyl)-9H-pyrimido[4,5-b]indol-4-amine ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3736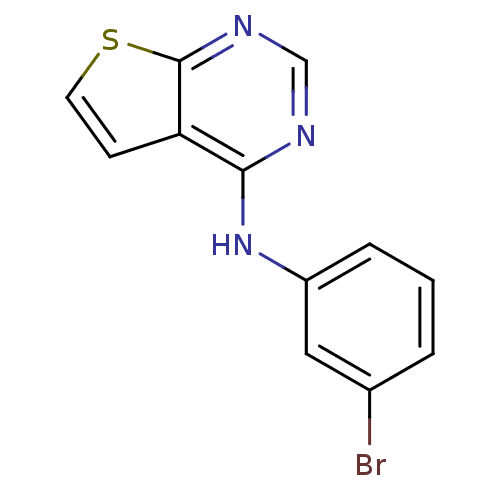 (Anilinopyrimidine deriv. 4 | N-(3-bromophenyl)thie...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3785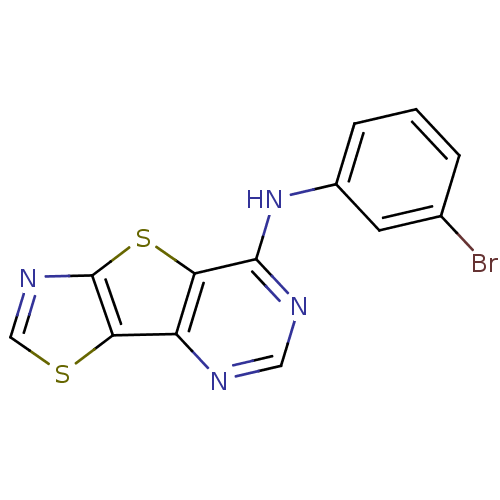 (N-(3-bromophenyl)-3,7-dithia-5,10,12-triazatricycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50075539 (3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3769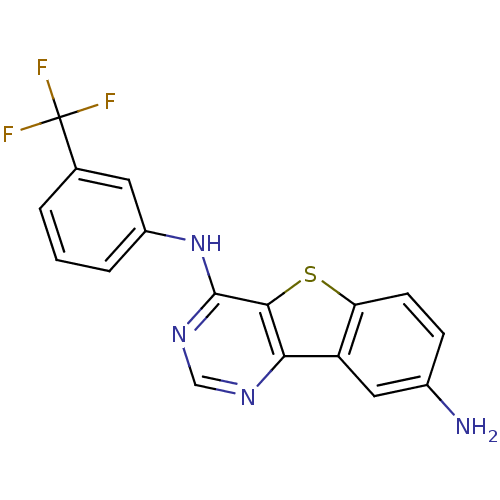 (6-N-[3-(trifluoromethyl)phenyl]-8-thia-3,5-diazatr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3771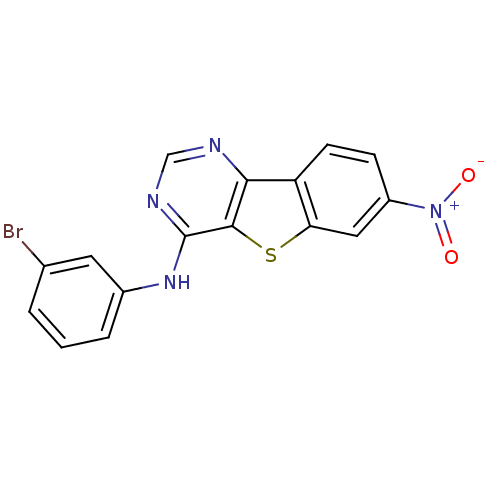 (Benzothieno[3,2-d]pyrimidine deriv. 62 | N-(3-brom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3763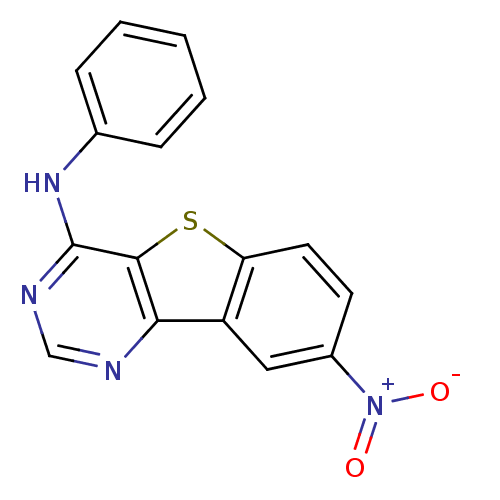 (12-nitro-N-phenyl-8-thia-3,5-diazatricyclo[7.4.0.0...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50044059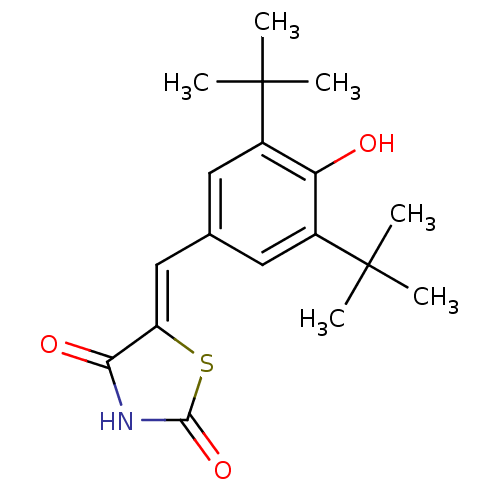 (5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-thiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50075526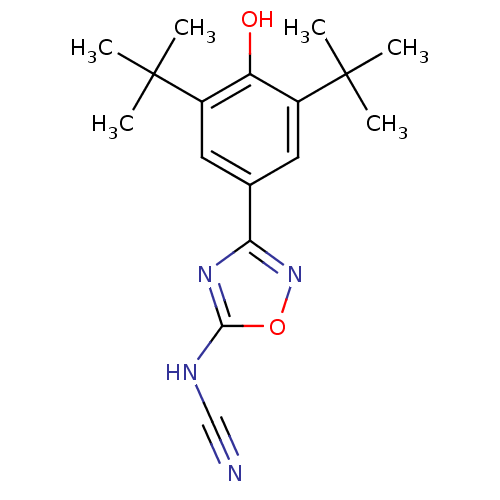 (3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]oxad...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) | J Med Chem 42: 1161-9 (1999) Article DOI: 10.1021/jm980570y BindingDB Entry DOI: 10.7270/Q2154G6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 433 total ) | Next | Last >> |