

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50345548 (CHEMBL1784538 | [13C,15N-Y,P,V]cyclic CRYPEVEIC | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Western Ontario Curated by ChEMBL | Assay Description Competitive inhibition of human GST-tagged-Pin1 PPIase activity using WFYpSPR-pNA as substrate by Michaelis-Menton equation | J Med Chem 54: 3854-65 (2011) Article DOI: 10.1021/jm200156c BindingDB Entry DOI: 10.7270/Q2DN45DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50345548 (CHEMBL1784538 | [13C,15N-Y,P,V]cyclic CRYPEVEIC | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Western Ontario Curated by ChEMBL | Assay Description Inhibition of human GST-tagged-Pin1 PPIase activity using Suc-AEPF-pNA as substrate by Michaelis-Menton equation | J Med Chem 54: 3854-65 (2011) Article DOI: 10.1021/jm200156c BindingDB Entry DOI: 10.7270/Q2DN45DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50345549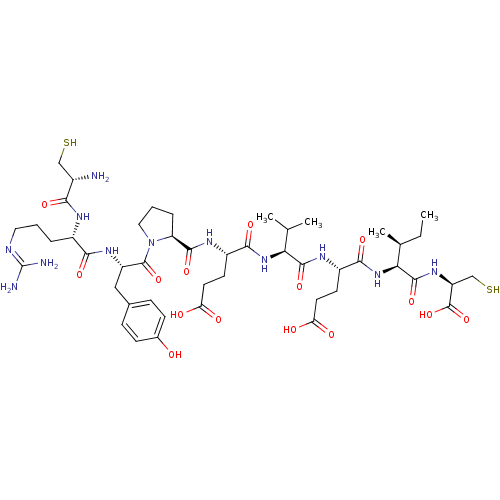 (CHEMBL1784539 | CRYPEVEIC) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Western Ontario Curated by ChEMBL | Assay Description Inhibition of human GST-tagged-Pin1 PPIase activity using Suc-AEPF-pNA as substrate by Michaelis-Menton equation | J Med Chem 54: 3854-65 (2011) Article DOI: 10.1021/jm200156c BindingDB Entry DOI: 10.7270/Q2DN45DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50156669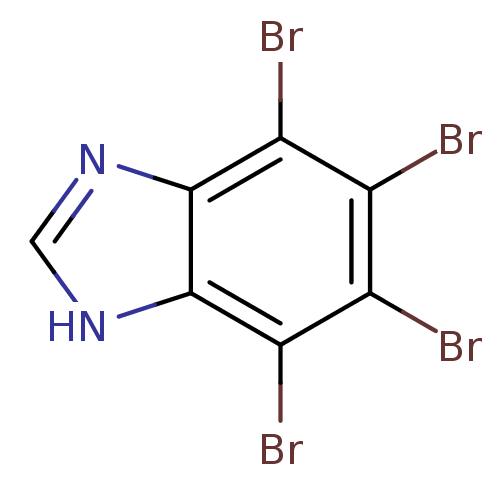 (4,5,6,7-TETRABROMO-BENZIMIDAZOLE | 4,5,6,7-tetrabr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Western Ontario | Assay Description To determine the IC50 value of each inhibitor, reactions were initiated by addition of 154 pM NQO2 to a reaction buffer containing 150 μM SCDP a... | Biochemistry 54: 47-59 (2015) Article DOI: 10.1021/bi500959t BindingDB Entry DOI: 10.7270/Q2BV7FC9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50156670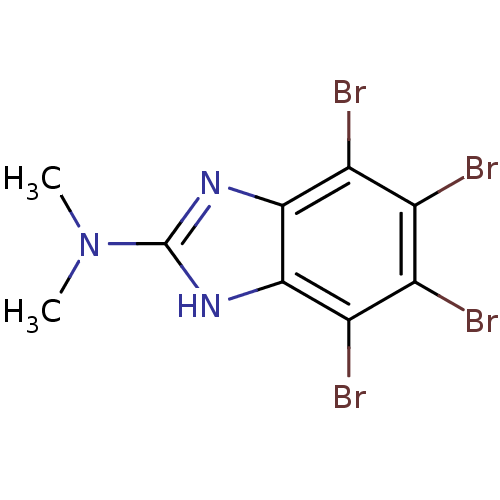 (2-(dimethylamino)-4,5,6,7-tetrabromo-1H-benzimidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Western Ontario | Assay Description To determine the IC50 value of each inhibitor, reactions were initiated by addition of 154 pM NQO2 to a reaction buffer containing 150 μM SCDP a... | Biochemistry 54: 47-59 (2015) Article DOI: 10.1021/bi500959t BindingDB Entry DOI: 10.7270/Q2BV7FC9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM11323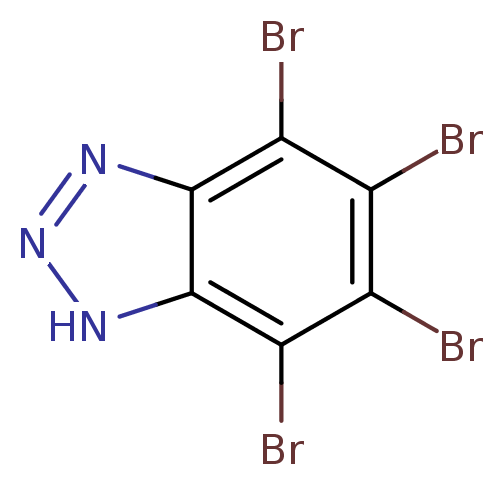 (4,5,6,7-tetrabromo-1H-1,2,3-benzotriazole | 4,5,6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Western Ontario | Assay Description To determine the IC50 value of each inhibitor, reactions were initiated by addition of 154 pM NQO2 to a reaction buffer containing 150 μM SCDP a... | Biochemistry 54: 47-59 (2015) Article DOI: 10.1021/bi500959t BindingDB Entry DOI: 10.7270/Q2BV7FC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50156670 (2-(dimethylamino)-4,5,6,7-tetrabromo-1H-benzimidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 36.4 | n/a | n/a | n/a | n/a | n/a |
University of Western Ontario | Assay Description To assess direct binding of inhibitors to oxidized NQO2 (NQO2ox), fluorescence quenching of FAD was monitored with an excitation wavelength of 350 nm... | Biochemistry 54: 47-59 (2015) Article DOI: 10.1021/bi500959t BindingDB Entry DOI: 10.7270/Q2BV7FC9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50156669 (4,5,6,7-TETRABROMO-BENZIMIDAZOLE | 4,5,6,7-tetrabr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a |
University of Western Ontario | Assay Description To assess direct binding of inhibitors to oxidized NQO2 (NQO2ox), fluorescence quenching of FAD was monitored with an excitation wavelength of 350 nm... | Biochemistry 54: 47-59 (2015) Article DOI: 10.1021/bi500959t BindingDB Entry DOI: 10.7270/Q2BV7FC9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM11323 (4,5,6,7-tetrabromo-1H-1,2,3-benzotriazole | 4,5,6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | 7.11E+3 | n/a | n/a | n/a | n/a | n/a |
University of Western Ontario | Assay Description To assess direct binding of inhibitors to oxidized NQO2 (NQO2ox), fluorescence quenching of FAD was monitored with an excitation wavelength of 350 nm... | Biochemistry 54: 47-59 (2015) Article DOI: 10.1021/bi500959t BindingDB Entry DOI: 10.7270/Q2BV7FC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||