

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109593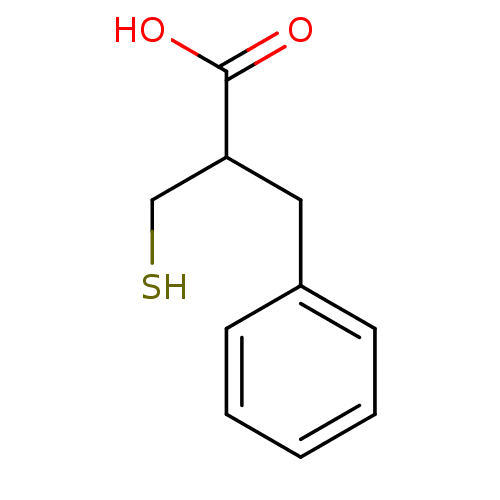 (2-Benzyl-3-mercapto-propionic acid | 2-Mercaptomet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279954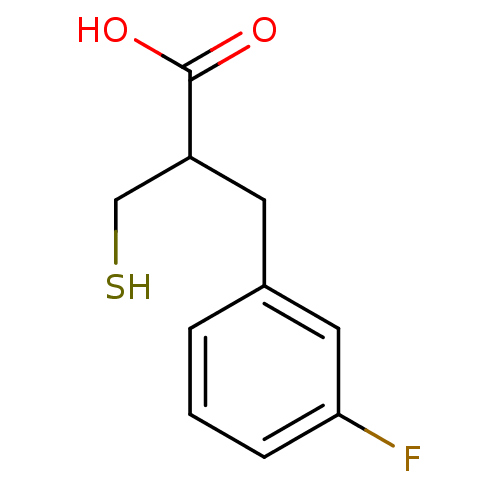 (3-(3-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279947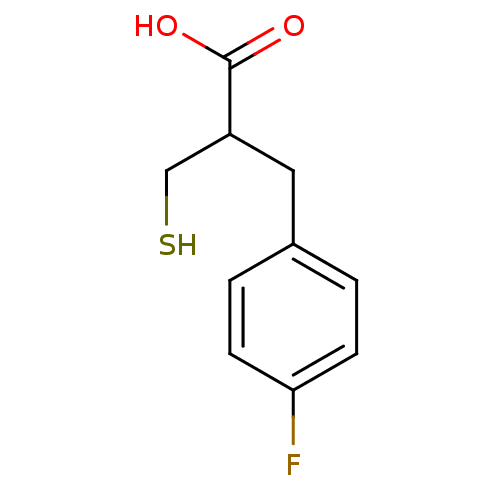 (3-(4-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279958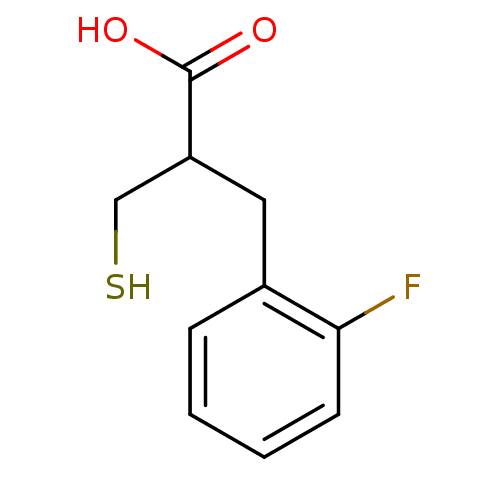 (3-(2-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279955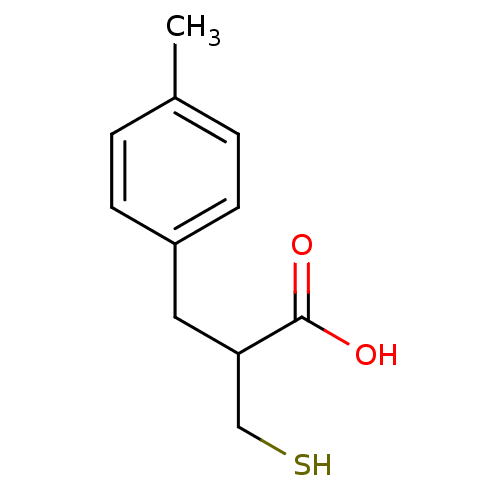 (2-Mercaptomethyl-3-p-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279956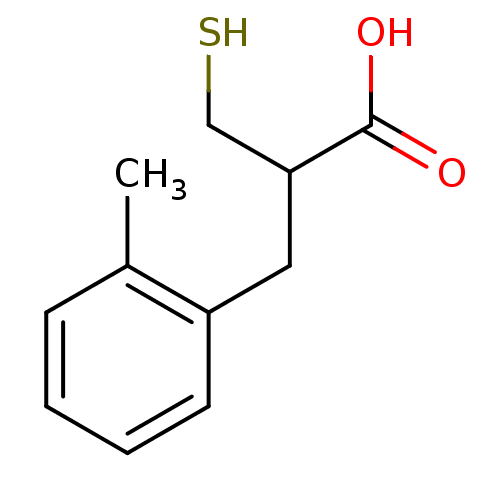 (2-Mercaptomethyl-3-o-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279953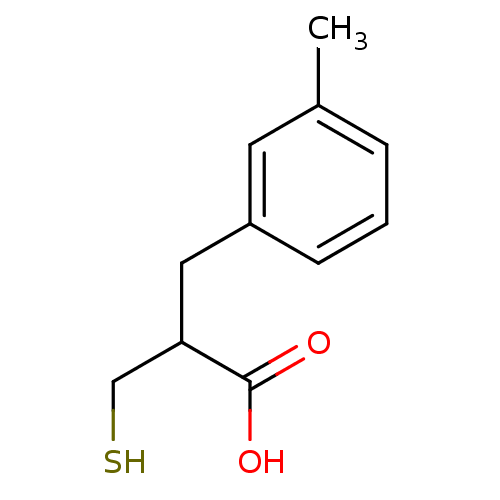 (2-Mercaptomethyl-3-m-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279949 (2-Mercaptomethyl-3-naphthalen-2-yl-propionic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279945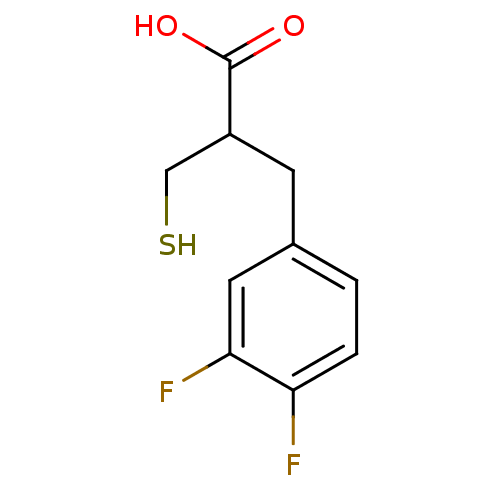 (3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279950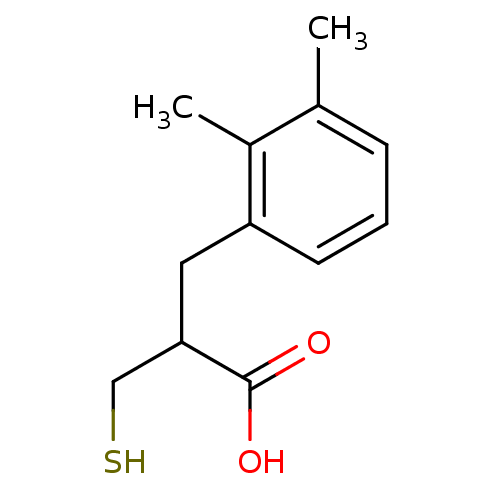 (3-(2,3-Dimethyl-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279957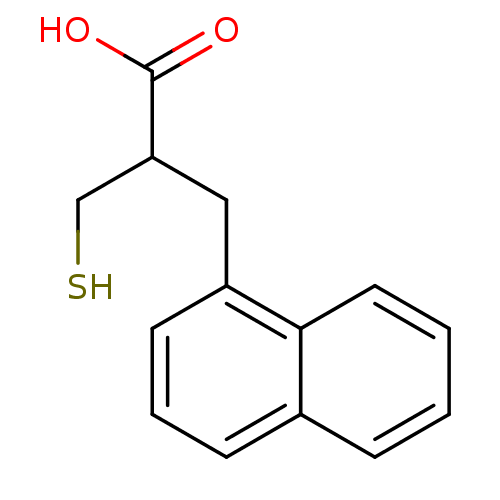 (2-Mercaptomethyl-3-naphthalen-1-yl-propionic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279952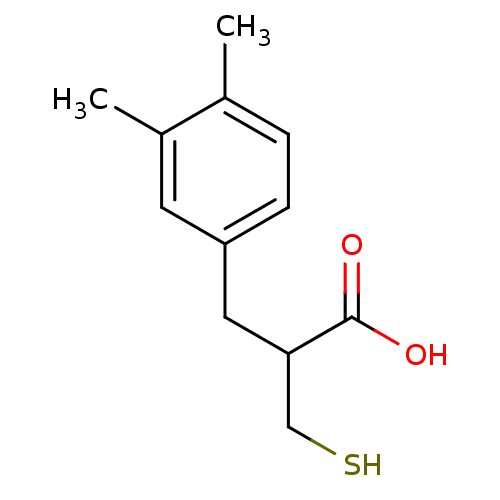 (3-(3,4-Dimethyl-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279946 (2-Mercaptomethyl-3-(3,4,5-trifluoro-phenyl)-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279951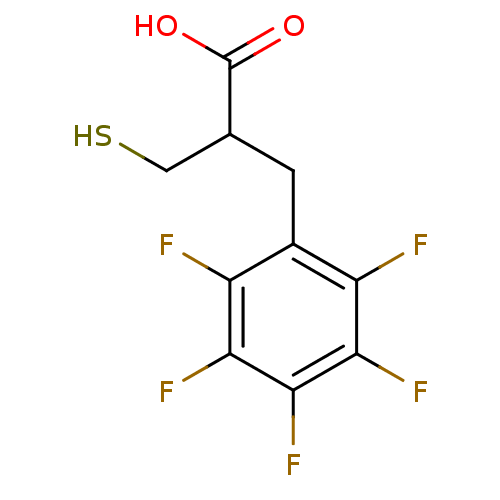 (2-Mercaptomethyl-3-pentafluorophenyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109602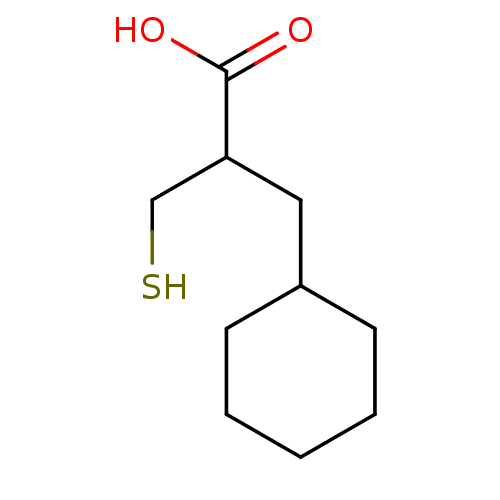 (3-Cyclohexyl-2-mercaptomethyl-propionic acid | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279959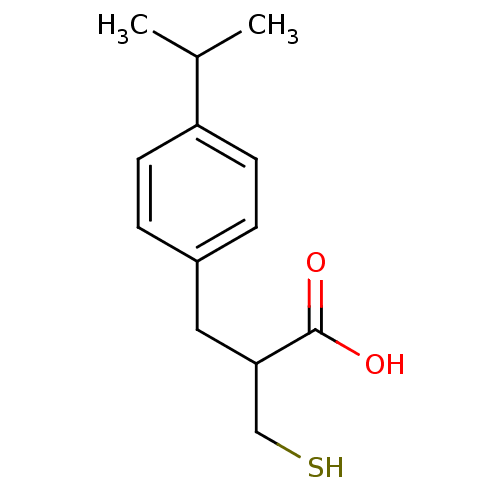 (3-(4-Isopropyl-phenyl)-2-mercaptomethyl-propionic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279948 (3-(4-tert-Butyl-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50537761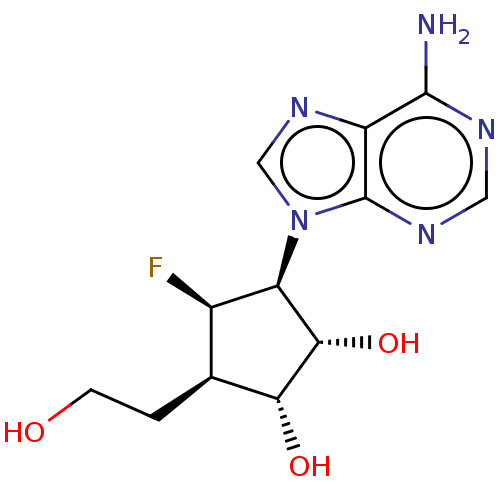 (CHEMBL4638533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50537760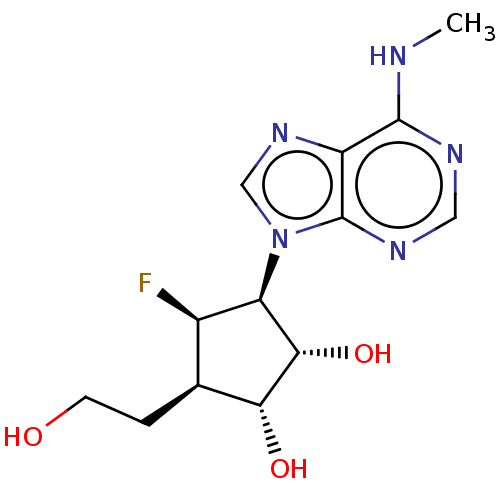 (CHEMBL4635734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50405728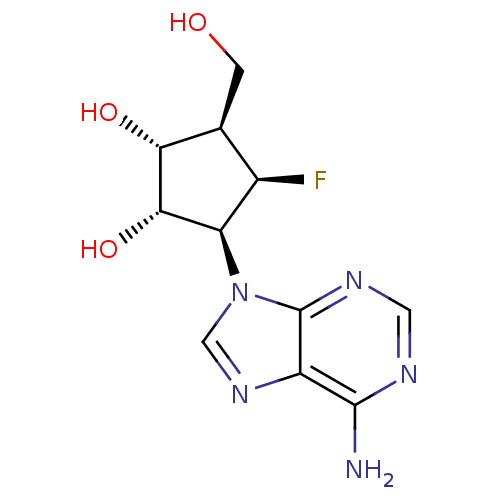 (CHEMBL2115462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50405728 (CHEMBL2115462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515221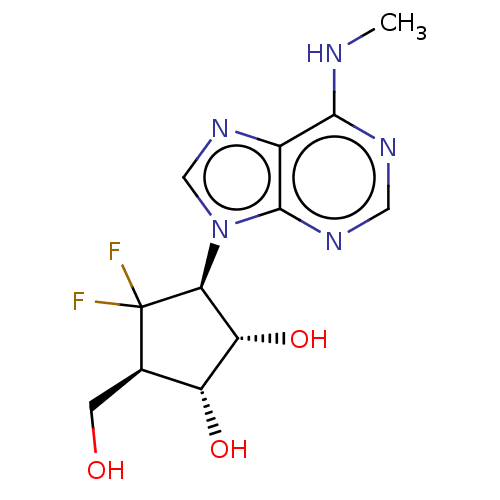 (CHEMBL4538845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515220 (CHEMBL4544781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515220 (CHEMBL4544781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50088426 ((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50537763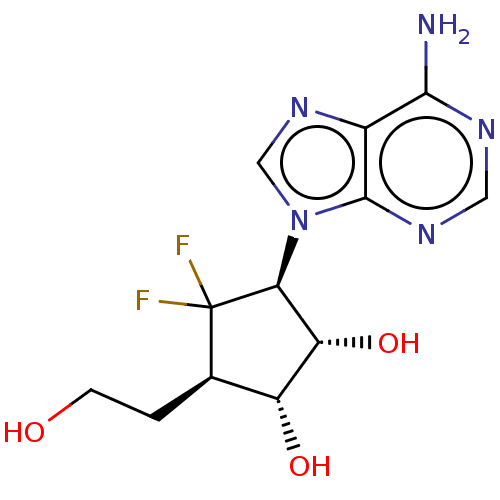 (CHEMBL4644901) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50537764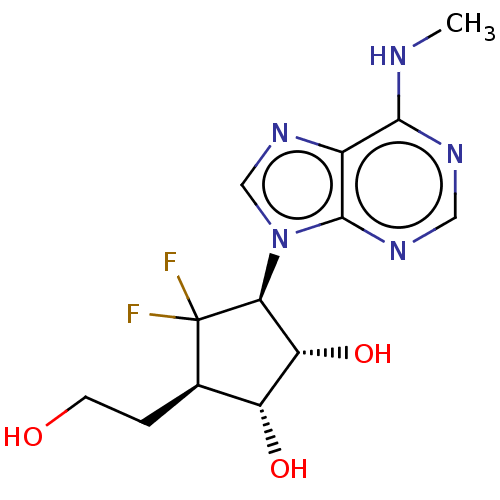 (CHEMBL4637324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515227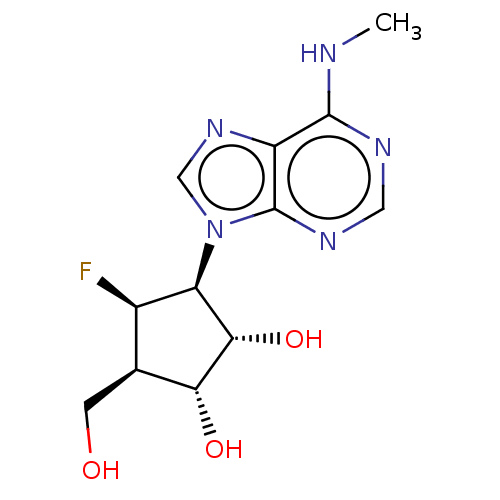 (CHEMBL4465968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50504578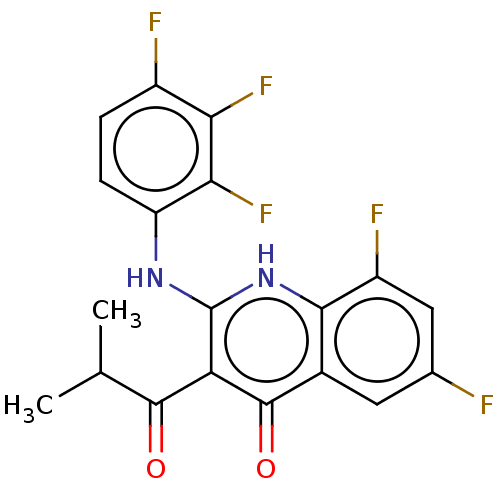 (CHEMBL4526734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126727 BindingDB Entry DOI: 10.7270/Q23J3H7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50405727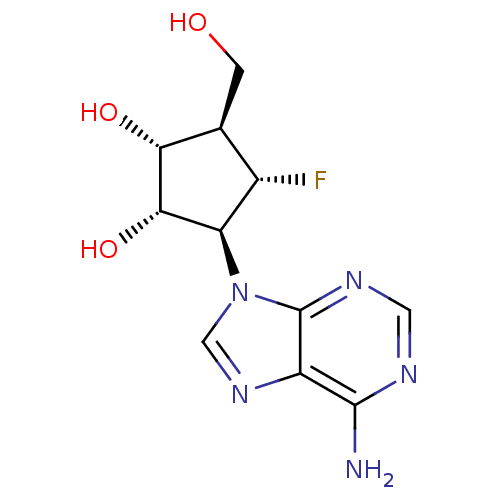 (CHEMBL2115031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515222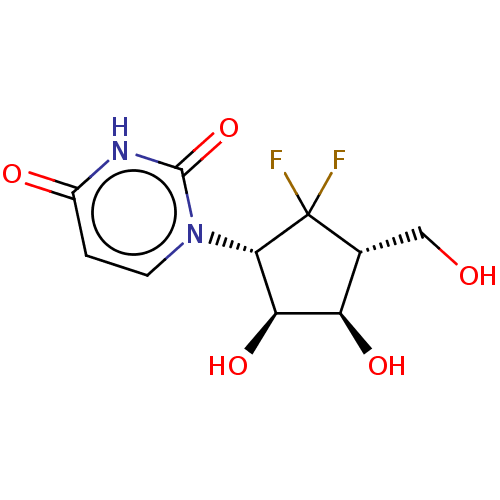 (CHEMBL4589829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515223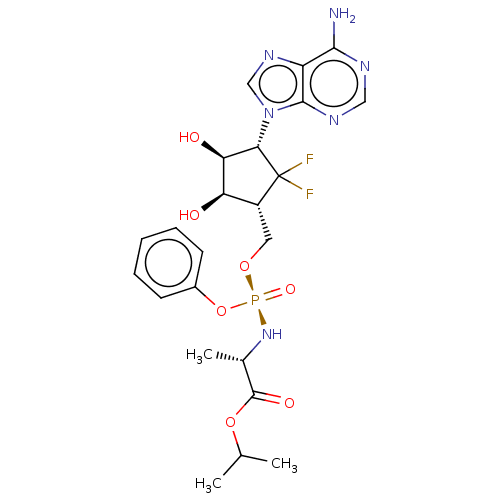 (CHEMBL4522602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515224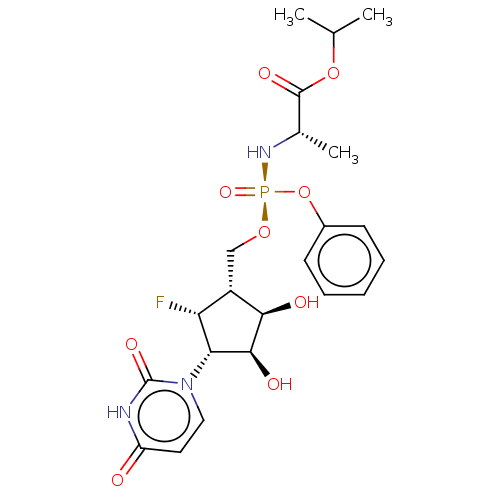 (CHEMBL4473818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50613929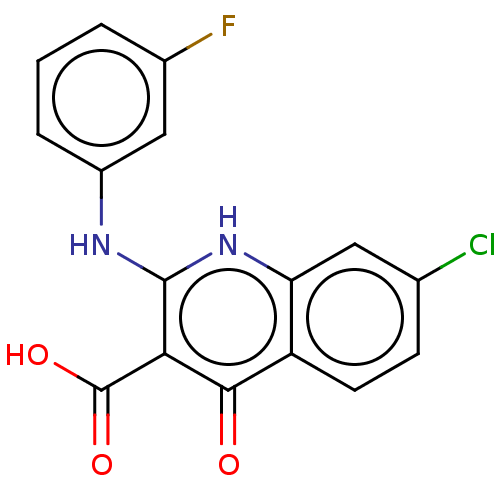 (CHEMBL5270848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50537762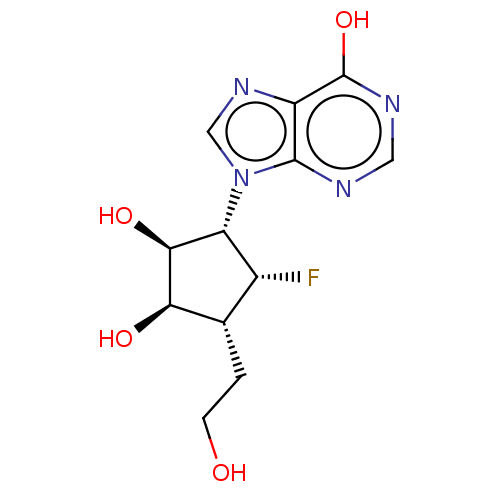 (CHEMBL4640283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111956 BindingDB Entry DOI: 10.7270/Q24F1V8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515219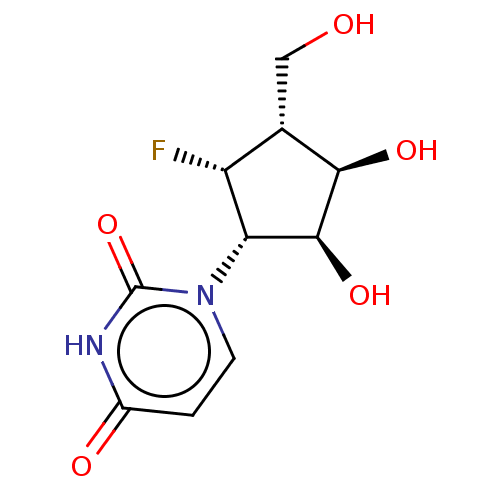 (CHEMBL4456225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515228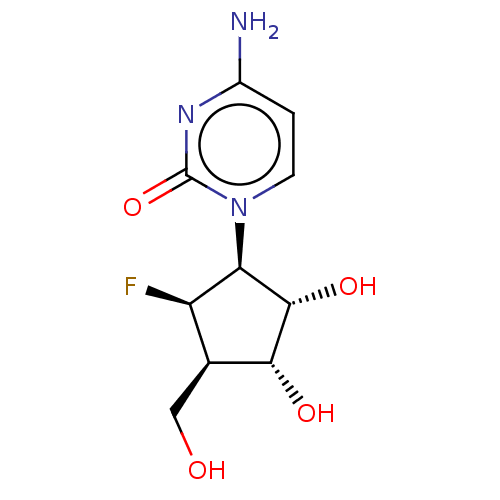 (CHEMBL4591427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515218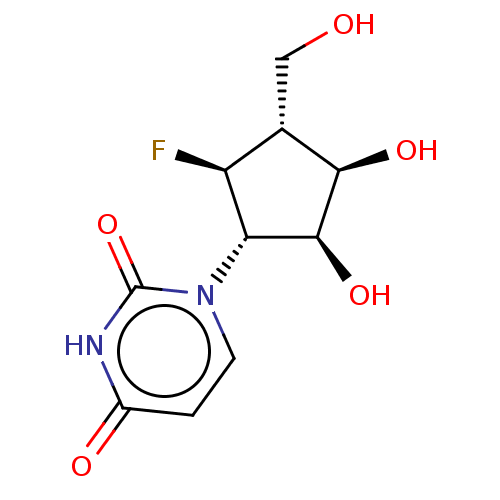 (CHEMBL4538994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515226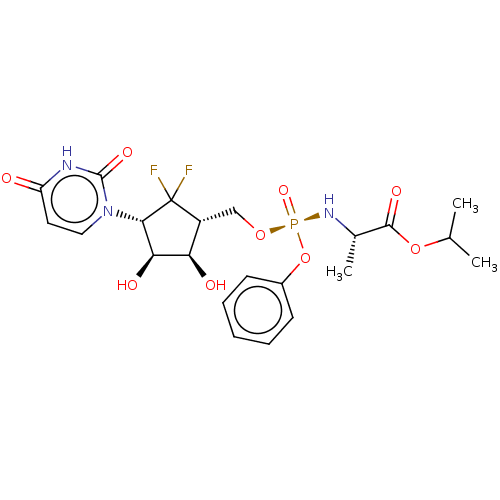 (CHEMBL4476535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50515225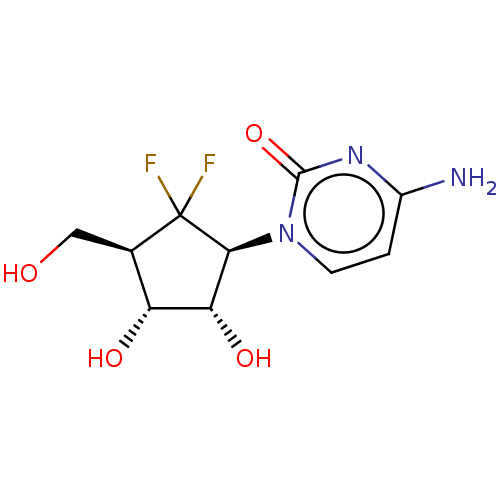 (CHEMBL4464364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human placental SAH hydrolase expressed in Escherichia coli JM109 using SAH as substrate preincubated for 10 mins followed by SAH addit... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515218 (CHEMBL4538994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515227 (CHEMBL4465968) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515223 (CHEMBL4522602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515220 (CHEMBL4544781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515222 (CHEMBL4589829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50088426 ((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515226 (CHEMBL4476535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515221 (CHEMBL4538845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50405727 (CHEMBL2115031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Zika virus) | BDBM50515219 (CHEMBL4456225) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent RNA polymerase in ZIKV SL0612 infected in African green monkey Vero cells assessed as reduction in virus-induced cytopath... | J Med Chem 62: 6346-6362 (2019) Article DOI: 10.1021/acs.jmedchem.9b00781 BindingDB Entry DOI: 10.7270/Q25Q50GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |