 Found 16 hits with Last Name = 'simpson' and Initial = 'pt'
Found 16 hits with Last Name = 'simpson' and Initial = 'pt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119291
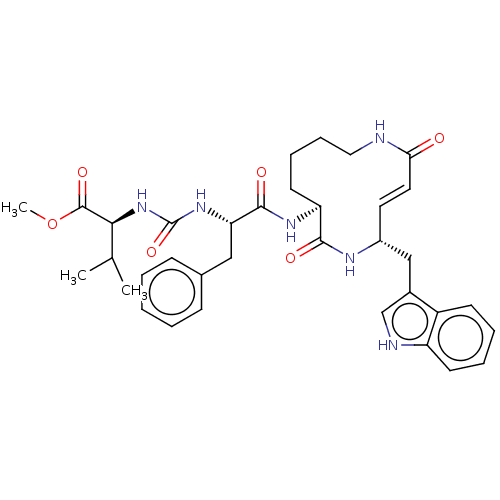
(CHEMBL3613827)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C |r,t:29| Show InChI InChI=1S/C35H44N6O6/c1-22(2)31(34(45)47-3)41-35(46)40-29(19-23-11-5-4-6-12-23)33(44)39-28-15-9-10-18-36-30(42)17-16-25(38-32(28)43)20-24-21-37-27-14-8-7-13-26(24)27/h4-8,11-14,16-17,21-22,25,28-29,31,37H,9-10,15,18-20H2,1-3H3,(H,36,42)(H,38,43)(H,39,44)(H2,40,41,46)/b17-16+/t25-,28+,29+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119290
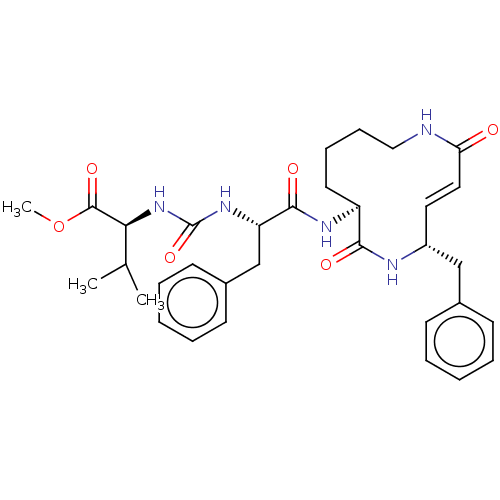
(CHEMBL3613826)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2)NC1=O)C(C)C |r,t:29| Show InChI InChI=1S/C33H43N5O6/c1-22(2)29(32(42)44-3)38-33(43)37-27(21-24-14-8-5-9-15-24)31(41)36-26-16-10-11-19-34-28(39)18-17-25(35-30(26)40)20-23-12-6-4-7-13-23/h4-9,12-15,17-18,22,25-27,29H,10-11,16,19-21H2,1-3H3,(H,34,39)(H,35,40)(H,36,41)(H2,37,38,43)/b18-17+/t25-,26+,27+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119297

(CHEMBL3613817)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(F)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42FN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-11-6-7-14-31-23(36)13-12-21(32-26(22)37)16-19-9-8-10-20(30)15-19/h8-10,12-13,15,17-18,21-22,24-25H,6-7,11,14,16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b13-12+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119294
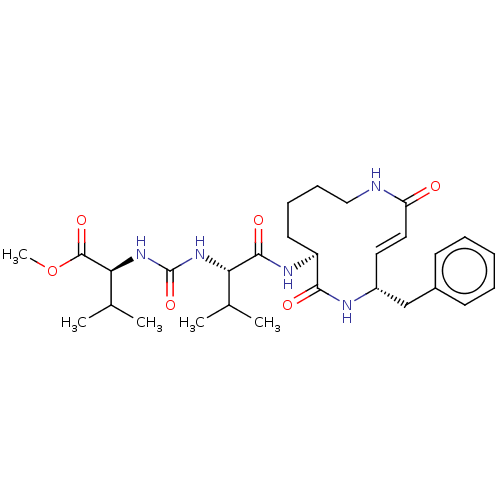
(CHEMBL3613814)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H43N5O6/c1-18(2)24(33-29(39)34-25(19(3)4)28(38)40-5)27(37)32-22-13-9-10-16-30-23(35)15-14-21(31-26(22)36)17-20-11-7-6-8-12-20/h6-8,11-12,14-15,18-19,21-22,24-25H,9-10,13,16-17H2,1-5H3,(H,30,35)(H,31,36)(H,32,37)(H2,33,34,39)/b15-14+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 743 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119296
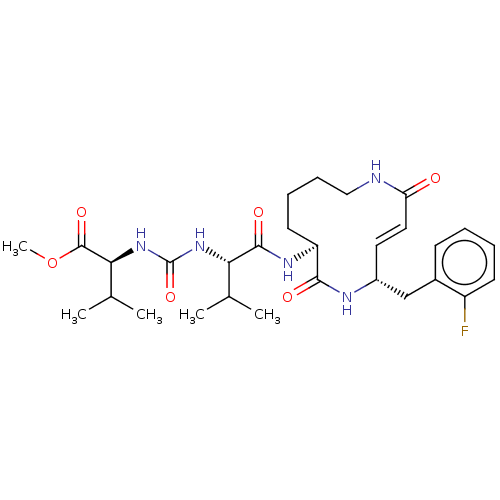
(CHEMBL3613816)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2F)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42FN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-12-8-9-15-31-23(36)14-13-20(32-26(22)37)16-19-10-6-7-11-21(19)30/h6-7,10-11,13-14,17-18,20,22,24-25H,8-9,12,15-16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t20-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 906 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119289

(CHEMBL3613825)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@@H](NC1=O)C(C)C)C(C)C |r,t:29| Show InChI InChI=1S/C29H43N5O6/c1-18(2)21-14-15-24(35)30-16-10-9-13-22(26(36)31-21)32-27(37)23(17-20-11-7-6-8-12-20)33-29(39)34-25(19(3)4)28(38)40-5/h6-8,11-12,14-15,18-19,21-23,25H,9-10,13,16-17H2,1-5H3,(H,30,35)(H,31,36)(H,32,37)(H2,33,34,39)/b15-14+/t21-,22+,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119299

(CHEMBL3613818)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cc(F)cc(F)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H41F2N5O6/c1-16(2)24(35-29(41)36-25(17(3)4)28(40)42-5)27(39)34-22-8-6-7-11-32-23(37)10-9-21(33-26(22)38)14-18-12-19(30)15-20(31)13-18/h9-10,12-13,15-17,21-22,24-25H,6-8,11,14H2,1-5H3,(H,32,37)(H,33,38)(H,34,39)(H2,35,36,41)/b10-9+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 952 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119301
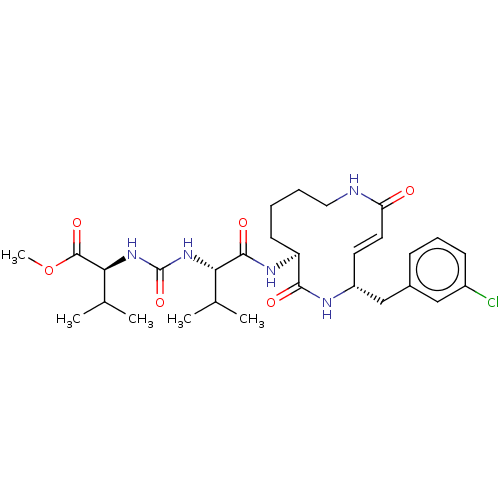
(CHEMBL3613820)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(Cl)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42ClN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-11-6-7-14-31-23(36)13-12-21(32-26(22)37)16-19-9-8-10-20(30)15-19/h8-10,12-13,15,17-18,21-22,24-25H,6-7,11,14,16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b13-12+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 963 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119304

(CHEMBL3613823)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccc(C)cc2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C30H45N5O6/c1-18(2)25(34-30(40)35-26(19(3)4)29(39)41-6)28(38)33-23-9-7-8-16-31-24(36)15-14-22(32-27(23)37)17-21-12-10-20(5)11-13-21/h10-15,18-19,22-23,25-26H,7-9,16-17H2,1-6H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b15-14+/t22-,23+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119302
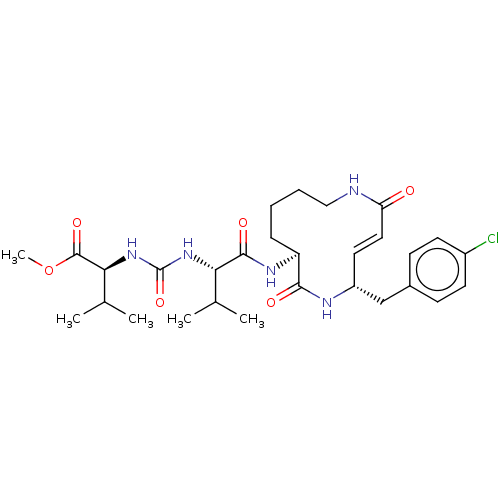
(CHEMBL3613821)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccc(Cl)cc2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42ClN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-8-6-7-15-31-23(36)14-13-21(32-26(22)37)16-19-9-11-20(30)12-10-19/h9-14,17-18,21-22,24-25H,6-8,15-16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119288

(CHEMBL3613824)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C31H44N6O6/c1-18(2)26(36-31(42)37-27(19(3)4)30(41)43-5)29(40)35-24-12-8-9-15-32-25(38)14-13-21(34-28(24)39)16-20-17-33-23-11-7-6-10-22(20)23/h6-7,10-11,13-14,17-19,21,24,26-27,33H,8-9,12,15-16H2,1-5H3,(H,32,38)(H,34,39)(H,35,40)(H2,36,37,42)/b14-13+/t21-,24+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119300

(CHEMBL3613819)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2Cl)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42ClN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-12-8-9-15-31-23(36)14-13-20(32-26(22)37)16-19-10-6-7-11-21(19)30/h6-7,10-11,13-14,17-18,20,22,24-25H,8-9,12,15-16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t20-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119292
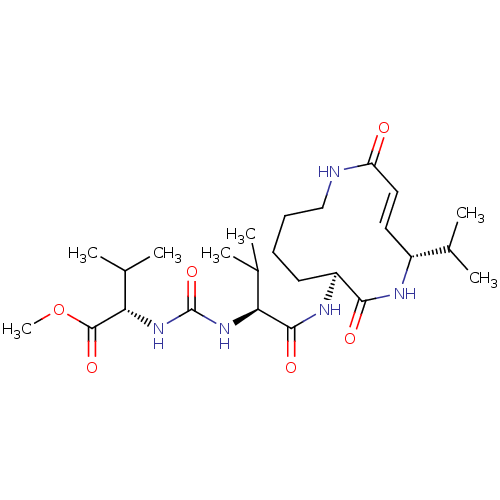
(CHEMBL3613812)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@@H](NC1=O)C(C)C)C(C)C |r,t:24| Show InChI InChI=1S/C25H43N5O6/c1-14(2)17-11-12-19(31)26-13-9-8-10-18(22(32)27-17)28-23(33)20(15(3)4)29-25(35)30-21(16(5)6)24(34)36-7/h11-12,14-18,20-21H,8-10,13H2,1-7H3,(H,26,31)(H,27,32)(H,28,33)(H2,29,30,35)/b12-11+/t17-,18+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119303

(CHEMBL3613822)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(C)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C30H45N5O6/c1-18(2)25(34-30(40)35-26(19(3)4)29(39)41-6)28(38)33-23-12-7-8-15-31-24(36)14-13-22(32-27(23)37)17-21-11-9-10-20(5)16-21/h9-11,13-14,16,18-19,22-23,25-26H,7-8,12,15,17H2,1-6H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t22-,23+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119293

(CHEMBL3613813)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](CC(C)C)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C26H45N5O6/c1-15(2)14-18-11-12-20(32)27-13-9-8-10-19(23(33)28-18)29-24(34)21(16(3)4)30-26(36)31-22(17(5)6)25(35)37-7/h11-12,15-19,21-22H,8-10,13-14H2,1-7H3,(H,27,32)(H,28,33)(H,29,34)(H2,30,31,36)/b12-11+/t18-,19+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119295
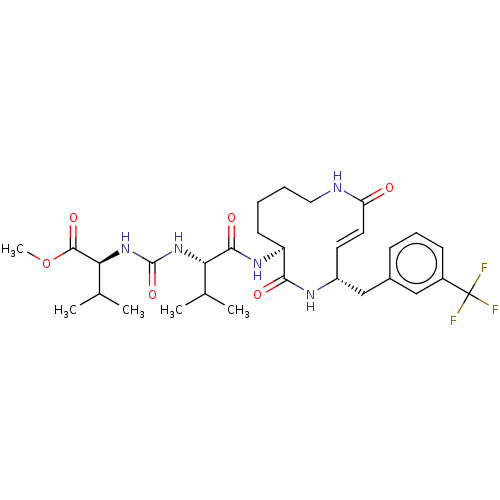
(CHEMBL3613815)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(c2)C(F)(F)F)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C30H42F3N5O6/c1-17(2)24(37-29(43)38-25(18(3)4)28(42)44-5)27(41)36-22-11-6-7-14-34-23(39)13-12-21(35-26(22)40)16-19-9-8-10-20(15-19)30(31,32)33/h8-10,12-13,15,17-18,21-22,24-25H,6-7,11,14,16H2,1-5H3,(H,34,39)(H,35,40)(H,36,41)(H2,37,38,43)/b13-12+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data