

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13614 (2-(carboxymethoxy)-5-[(2S)-2-(pentylcarbamoyl)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13611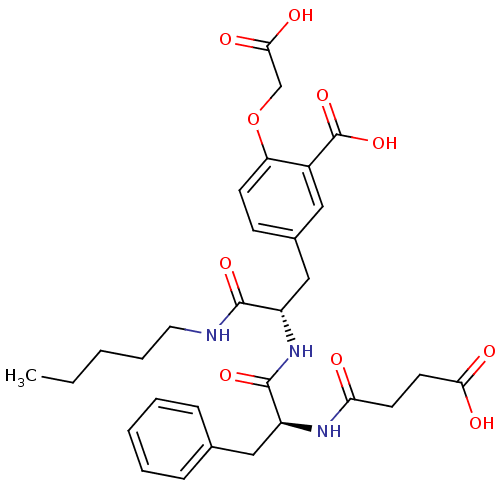 (2-(carboxymethoxy)-5-[(2S)-2-[(2S)-2-(3-formamidop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 250 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13609 (2-{4-[(2S)-2-[(2S)-2-(3-formamidopropanoic acid)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13610 (2-(carboxymethoxy)-5-[(2S)-2-(3-formamidopropanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13613 (2-{4-[(2S)-2-({[(1S)-1-carboxy-2-phenylethyl]carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 3.40E+3 | -30.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13606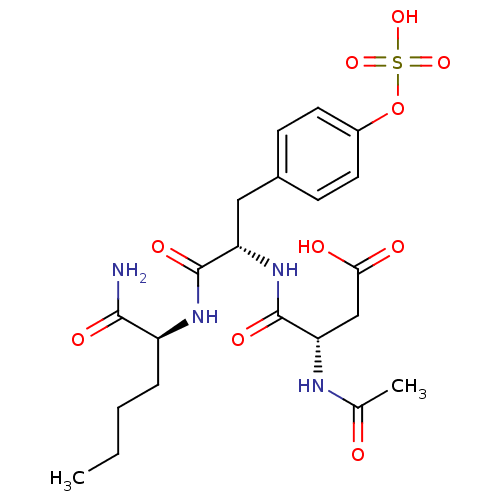 ((3S)-3-{[(1S)-1-{[(1S)-1-carbamoylpentyl]carbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13608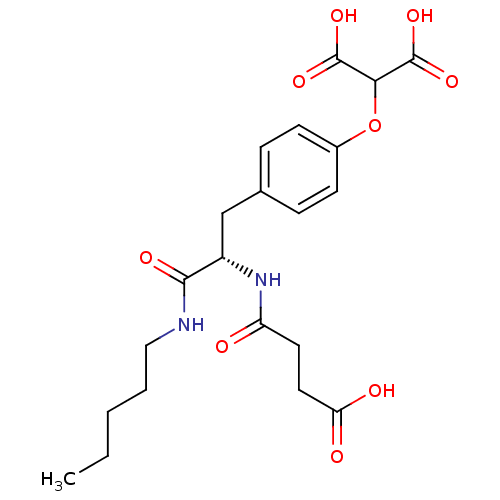 (2-{4-[(2S)-2-(3-formamidopropanoic acid)-2-(pentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13607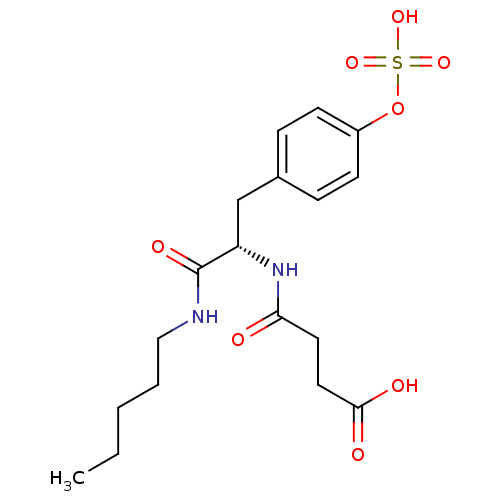 (3-{[(1S)-1-(pentylcarbamoyl)-2-[4-(sulfooxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM13612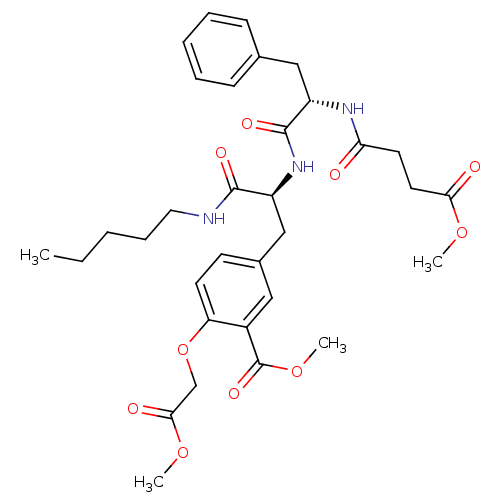 (Compound VII | methyl 2-(2-methoxy-2-oxoethoxy)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation | Assay Description Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... | Biochemistry 40: 5642-54 (2001) Article DOI: 10.1021/bi002865v BindingDB Entry DOI: 10.7270/Q2D50K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367718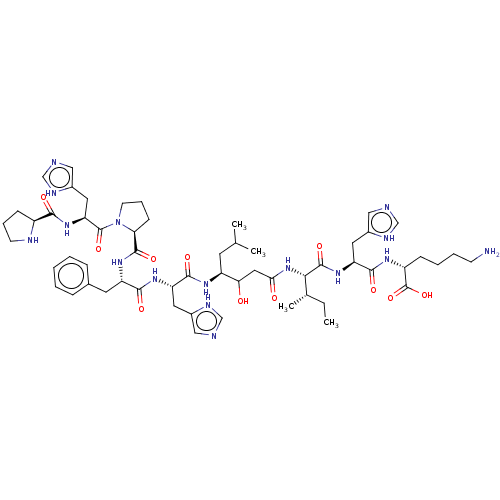 (CHEMBL3037868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367714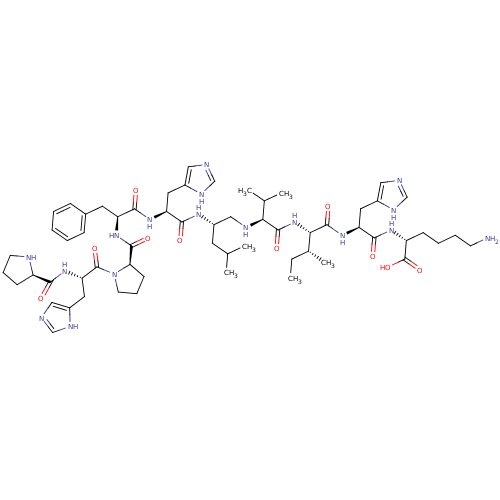 (CHEMBL1790111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367716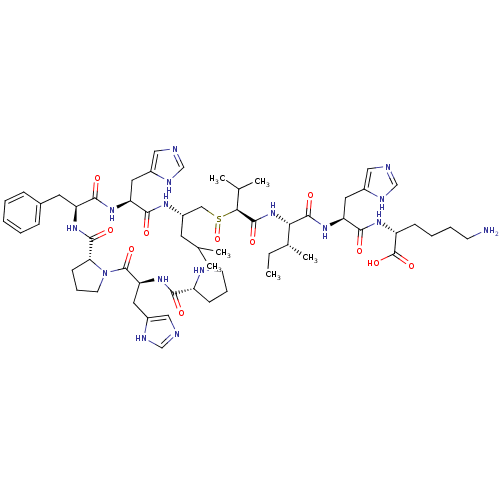 (CHEMBL1790120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367717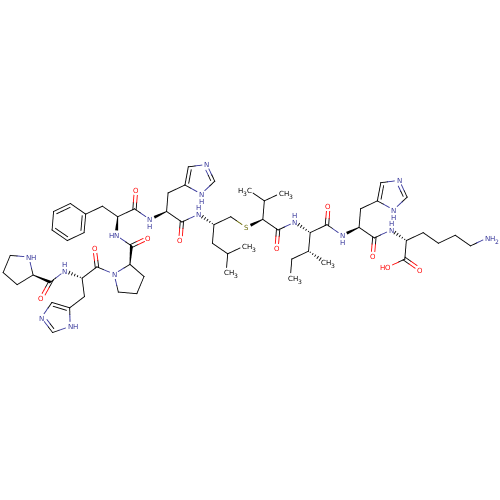 (CHEMBL1790115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367713 (CHEMBL1790122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022938 (CHEMBL438719 | H-Pro-His-Pro-Phe-His-Phe-Phe-Val-T...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367715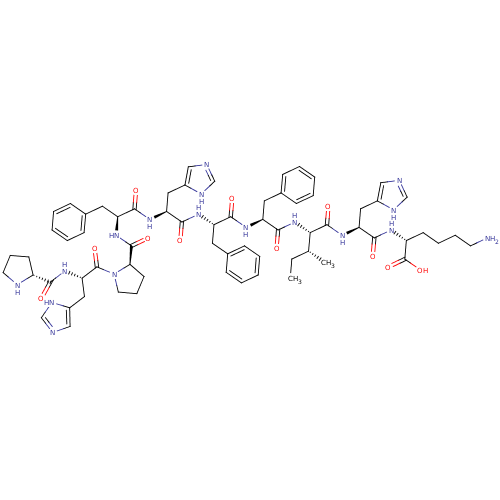 (CHEMBL1790121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||