

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523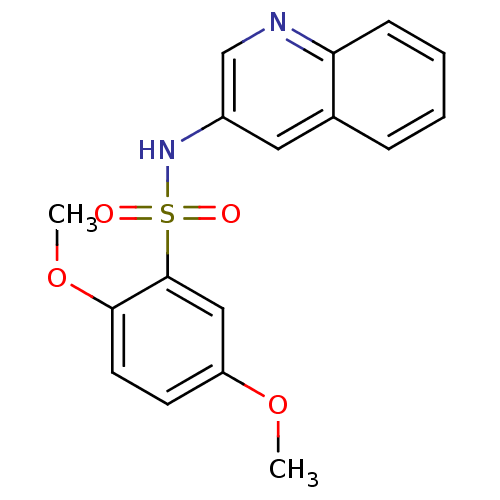 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP using CDP-star as substrate by non-competitive Lineweaver-Burk plot | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50440737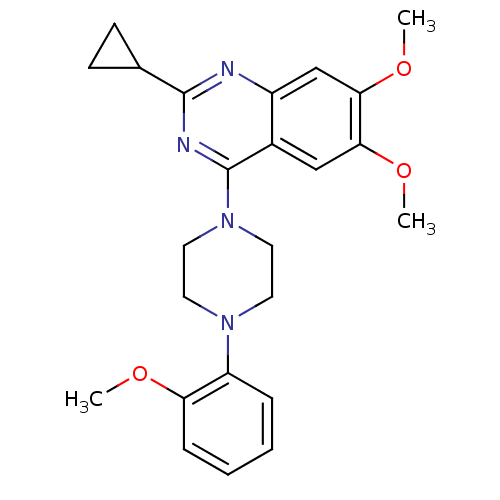 (CHEMBL2431120) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP using DEA as substrate by non-competitive Lineweaver-Burk plot | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to PBR receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of MOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DOR (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50444943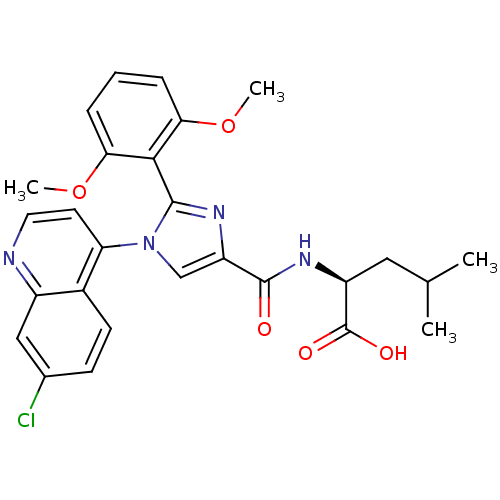 (CHEMBL3099773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of NTS1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50248035 ((2S)-2-(1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50444943 (CHEMBL3099773) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738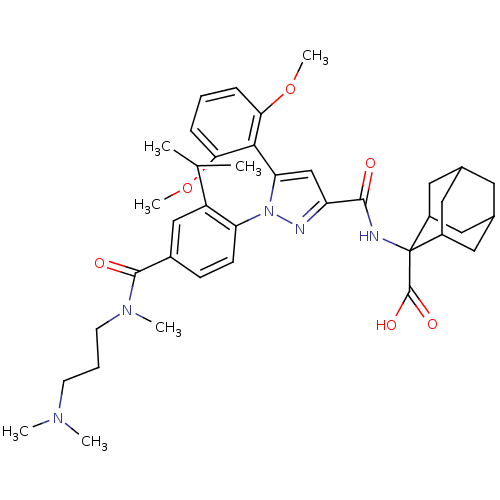 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-neurotensin from NTR1 (unknown origin) | Bioorg Med Chem Lett 24: 262-7 (2013) Article DOI: 10.1016/j.bmcl.2013.11.026 BindingDB Entry DOI: 10.7270/Q2NV9KQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-neurotensin from NTR1 in HUVEC after 1 hr by gamma counting analysis | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM85050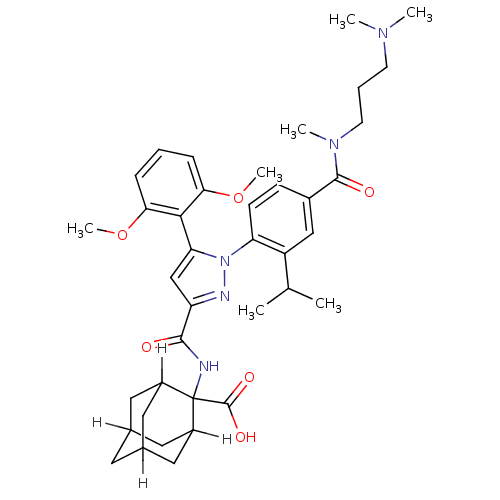 (CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at NTR1 (unknown origin) | J Med Chem 62: 8357-8363 (2019) Article DOI: 10.1021/acs.jmedchem.9b00340 BindingDB Entry DOI: 10.7270/Q2HQ438V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 2 (Homo sapiens (Human)) | BDBM62288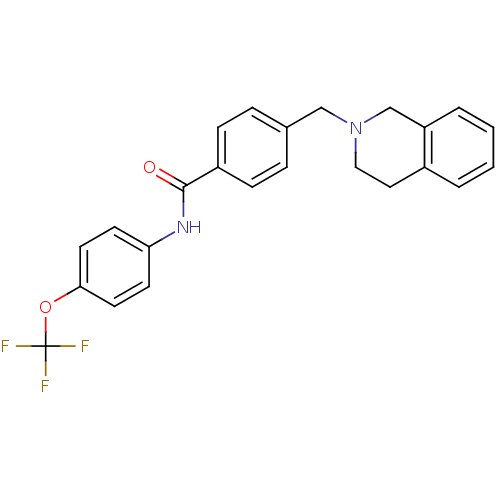 (4-(3,4-dihydro-1H-isoquinolin-2-ylmethyl)-N-[4-(tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-2 mediated NFkappaB activation in HEK293T cells assessed as inhibition of MDP-induced luciferase activity after 14 hrs by reporter ... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539483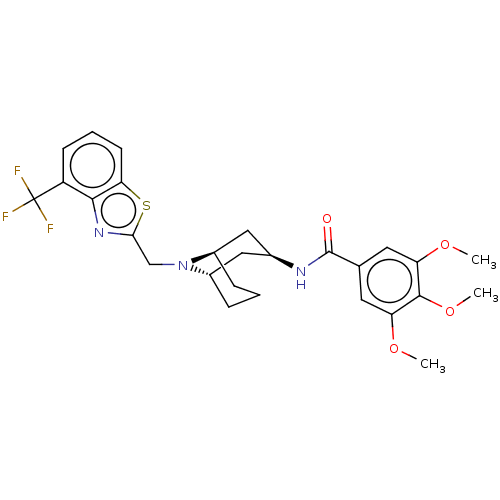 (CHEMBL4637126) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539483 (CHEMBL4637126) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NTR1 (unknown origin) expressed in human U2OS cells coexpressing beta-arrestin assessed as inhibition of ML314-induced effect ... | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM62288 (4-(3,4-dihydro-1H-isoquinolin-2-ylmethyl)-N-[4-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539482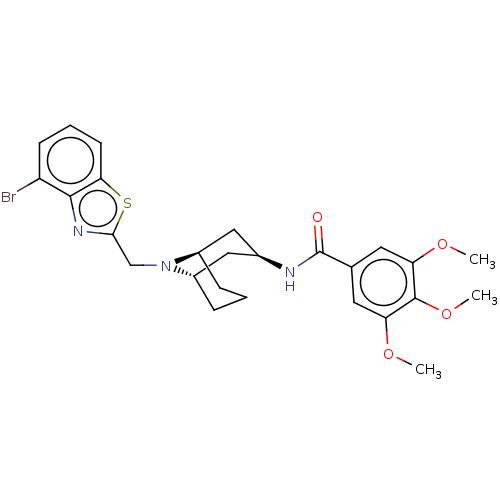 (CHEMBL4638895) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50248034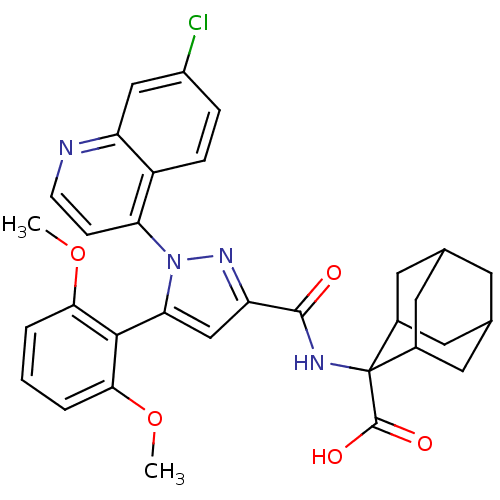 (2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Displacement of [125I]neurotensin from rat brain NTR1 | J Med Chem 62: 8357-8363 (2019) Article DOI: 10.1021/acs.jmedchem.9b00340 BindingDB Entry DOI: 10.7270/Q2HQ438V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM62170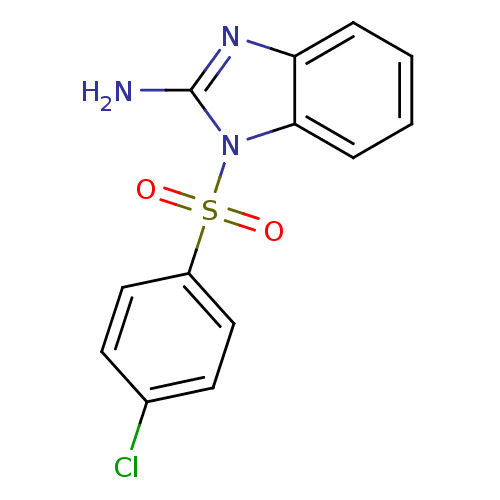 (1-(4-chlorophenyl)sulfonyl-2-benzimidazolamine | 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539479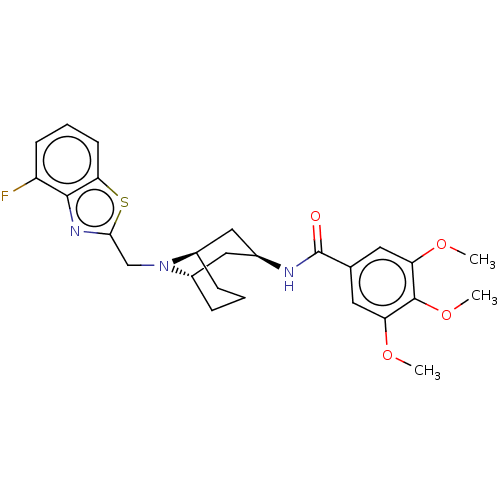 (CHEMBL4632425) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539449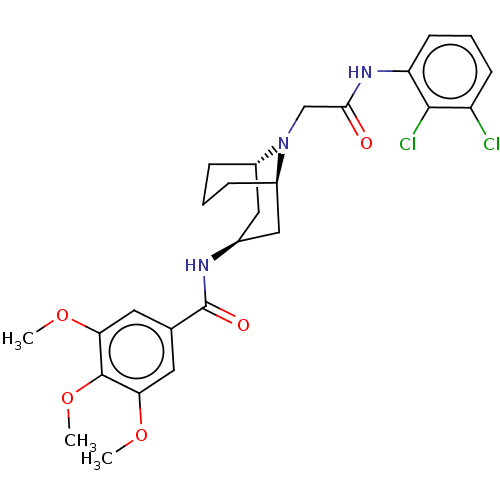 (CHEMBL4647701) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299534 (5-Bromo-2-methoxy-N-(quinolin-3-yl)benzenesulfonam...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP transfected in african green monkey COS1 cells by colorimetric assay | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539475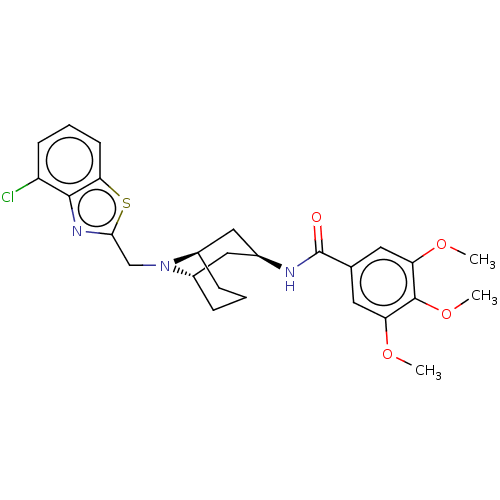 (CHEMBL4646120) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539451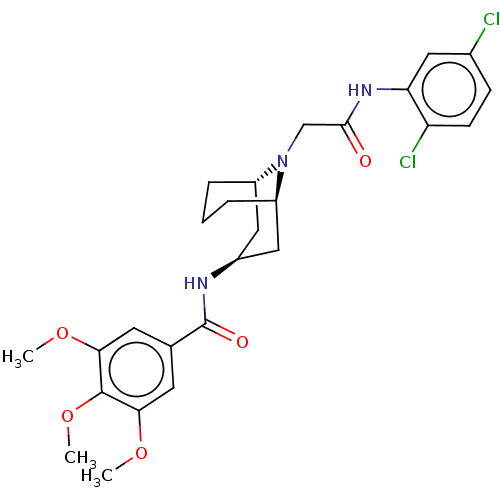 (CHEMBL4646569) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539457 (CHEMBL4645971) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoethanolamine/phosphocholine phosphatase (Homo sapiens (Human)) | BDBM45772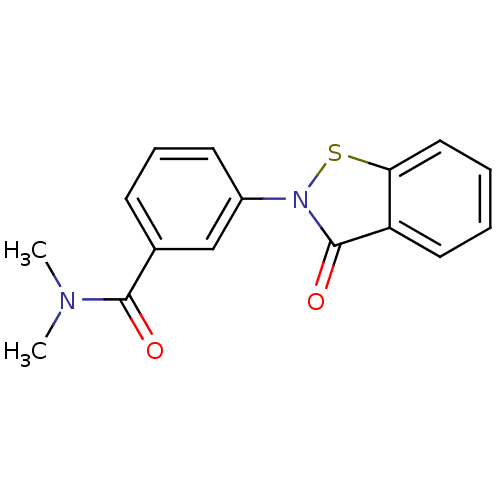 (3-(3-keto-1,2-benzothiazol-2-yl)-N,N-dimethyl-benz...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of PHOSPHO1 phosphoethanolamine activity (unknown origin) assessed as amount of inorganic phosphate release after 30 mins | Bioorg Med Chem Lett 24: 4308-11 (2014) Article DOI: 10.1016/j.bmcl.2014.07.013 BindingDB Entry DOI: 10.7270/Q2GX4D7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM54409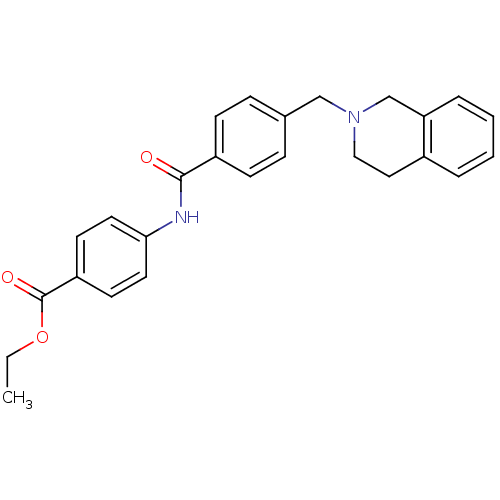 (4-[4-(3,4-Dihydro-1H-isoquinolin-2-ylmethyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539450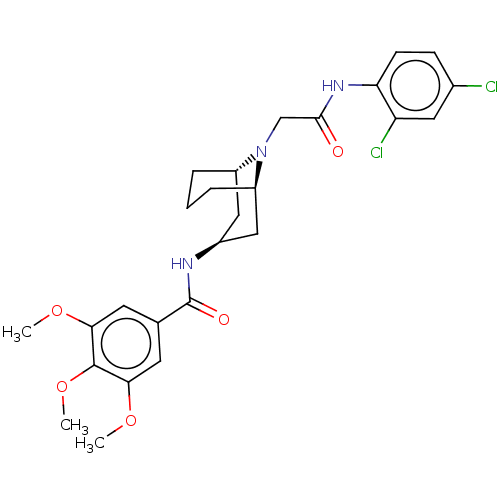 (CHEMBL4648997) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539440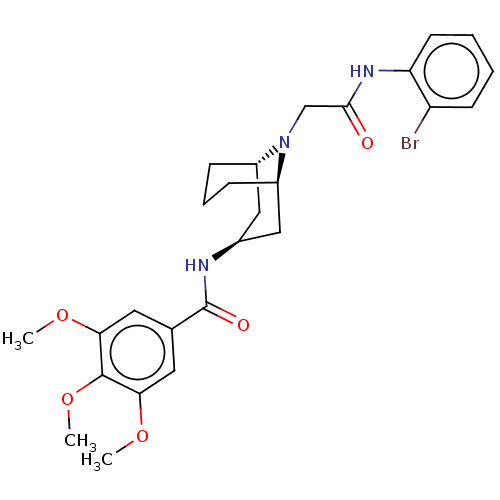 (CHEMBL4640388) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539476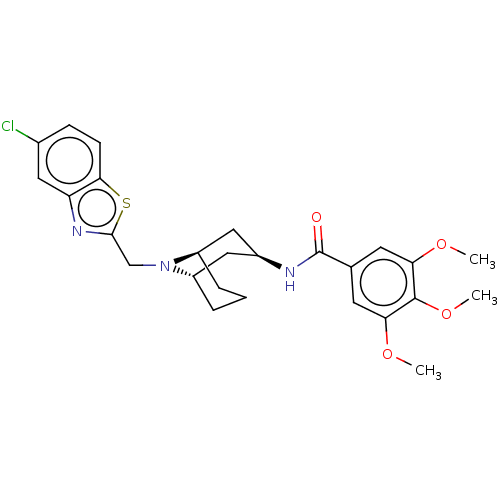 (CHEMBL4641681) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP transfected in african green monkey COS1 cells by colorimetric assay | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 2 (Homo sapiens (Human)) | BDBM62251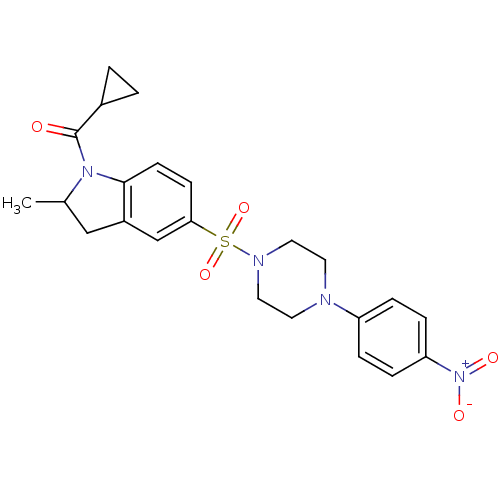 (MLS-0412140.0001 | cid_44182145 | cyclopropyl-[2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-2 mediated NFkappaB activation in HEK293T cells assessed as inhibition of MDP-induced luciferase activity after 14 hrs by reporter ... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP right after mixing with enzyme (EI 0:0) by colorimetric assay | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP after 1 hr incubation with enzyme post-dilution (EI 1:0) by colorimetric assay | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM62261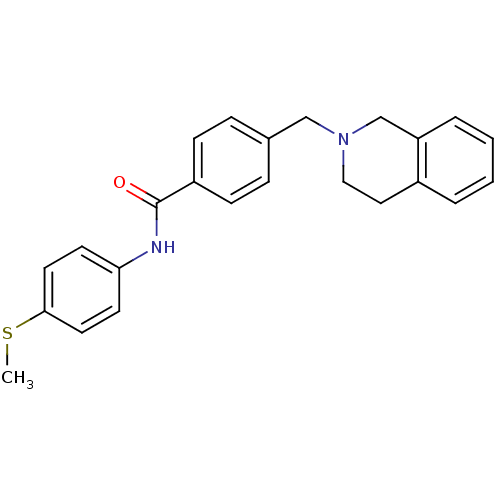 (4-(3,4-dihydro-1H-isoquinolin-2-ylmethyl)-N-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539480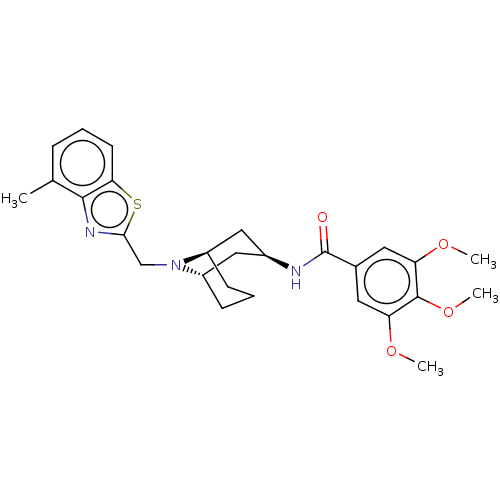 (CHEMBL4639692) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 1 (Homo sapiens (Human)) | BDBM62251 (MLS-0412140.0001 | cid_44182145 | cyclopropyl-[2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 2 (Homo sapiens (Human)) | BDBM62261 (4-(3,4-dihydro-1H-isoquinolin-2-ylmethyl)-N-(4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of NOD-2 mediated NFkappaB activation in HEK293T cells assessed as inhibition of MDP-induced luciferase activity after 14 hrs by reporter ... | ACS Med Chem Lett 2: 780-785 (2011) Article DOI: 10.1021/ml200158b BindingDB Entry DOI: 10.7270/Q2H9966Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539478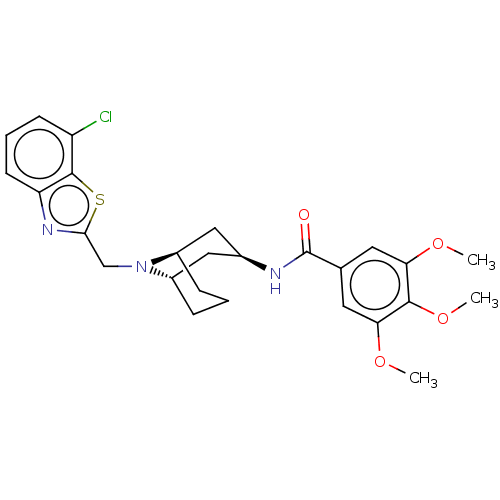 (CHEMBL4638493) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539477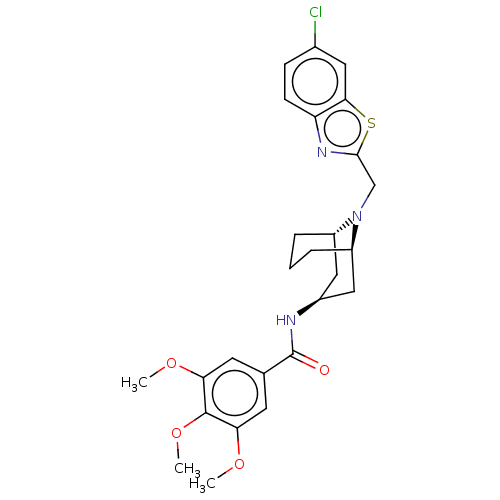 (CHEMBL4640319) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP after 1 hr pre-incubation with enzyme (EI 1:1) by colorimetric assay | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539470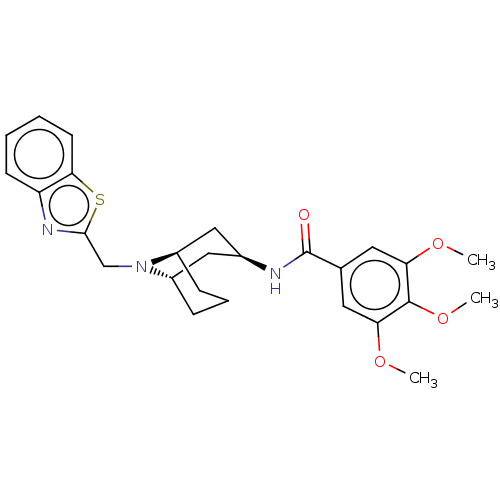 (CHEMBL4642230) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539454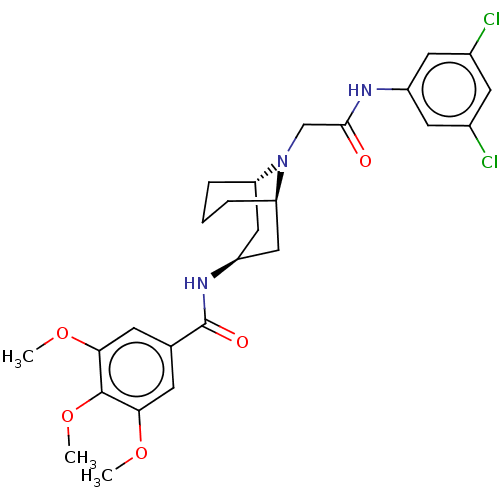 (CHEMBL4647206) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 26S proteasome non-ATPase regulatory subunit 14 (Homo sapiens (Human)) | BDBM224012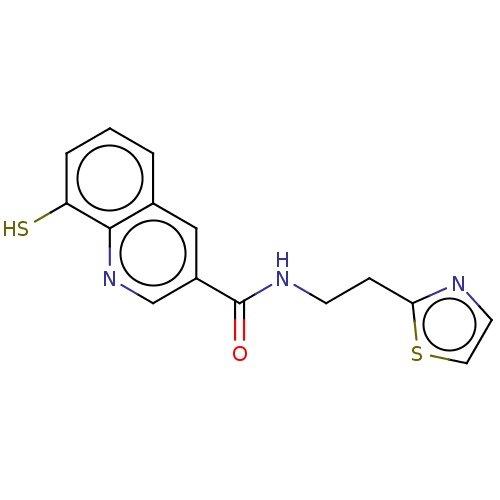 (Capzimin | US10005735, Compound 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.5 | 30 |
California Institute of Technology | Assay Description Fluorescence polarization assays were performed in low-volume 384-well solid black plates (Molecular Devices) in quadruplicate. The assays were perfo... | Nat Chem Biol 13: 486-493 (2017) Article DOI: 10.1038/nchembio.2326 BindingDB Entry DOI: 10.7270/Q24J0D0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539453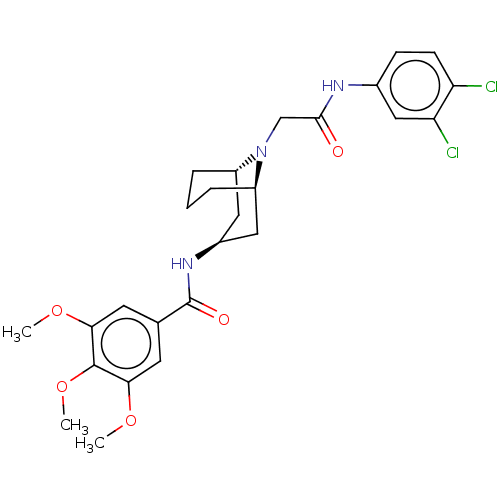 (CHEMBL4649351) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 6 (Homo sapiens) | BDBM50539446 (CHEMBL4645763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford Burnham Prebys Medical Discovery Institute Curated by ChEMBL | Assay Description Antagonist activity at Prolink-tagged human CXCR6 receptor assessed as inhibition of CXCL16-induced beta-arrestin recruitment by DiscoveRx cell based... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126899 BindingDB Entry DOI: 10.7270/Q2K077T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1203 total ) | Next | Last >> |