

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464108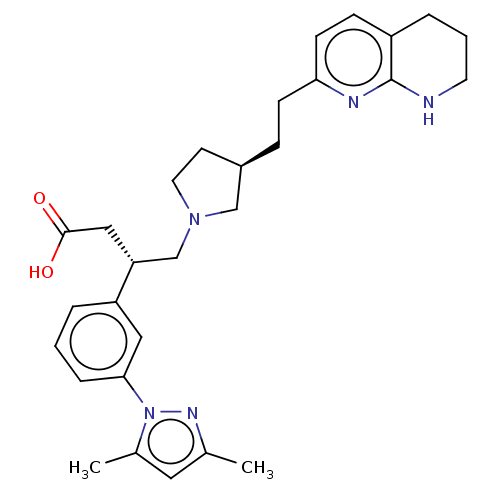 (CHEMBL4241824) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464104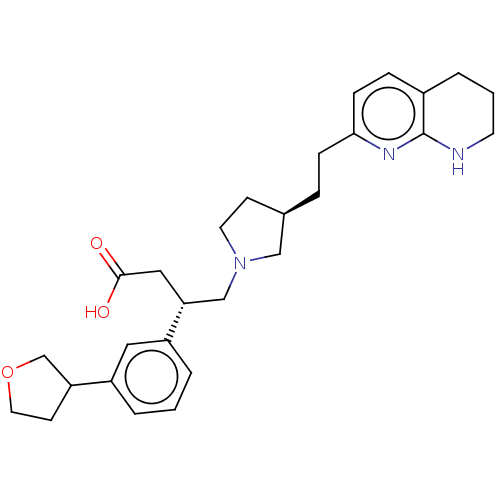 (CHEMBL4244784) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384817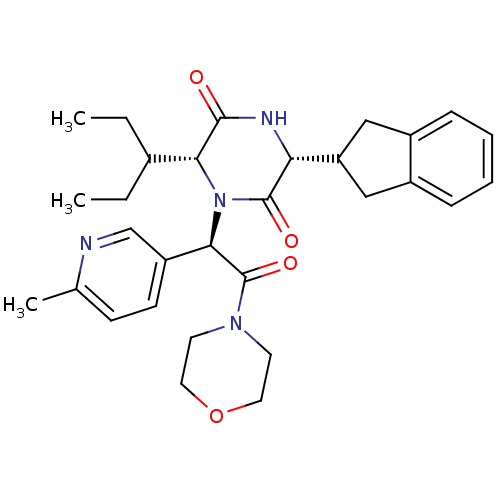 (CHEMBL2037514) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464110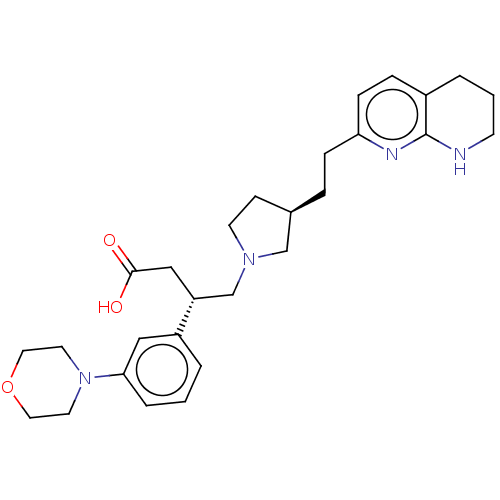 (CHEMBL4238909) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384800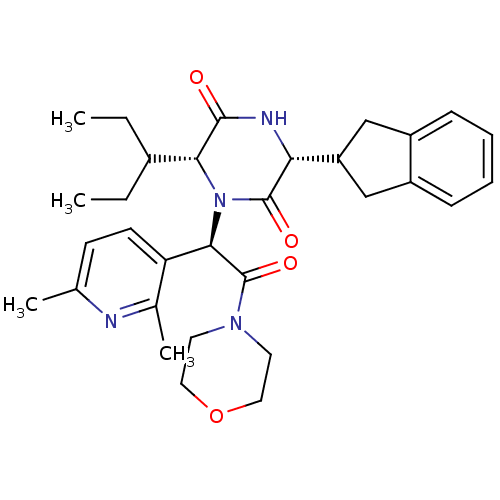 (CHEMBL2037517) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384816 (CHEMBL2037516) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464123 (CHEMBL4242263) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384823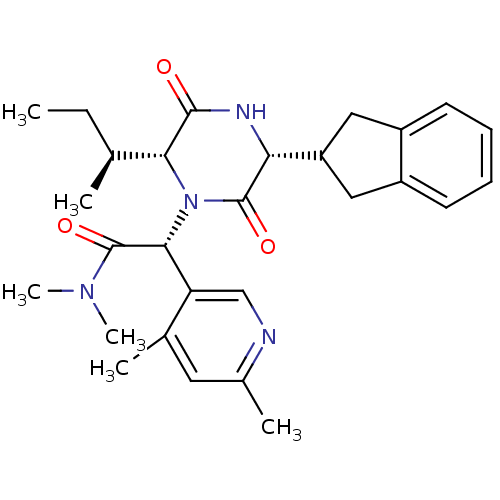 (CHEMBL2037507) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464119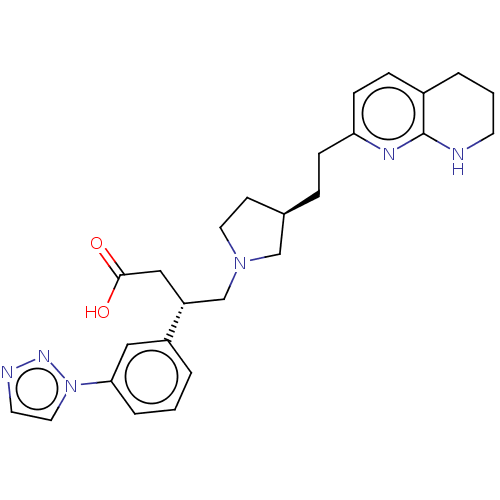 (CHEMBL4241584) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464118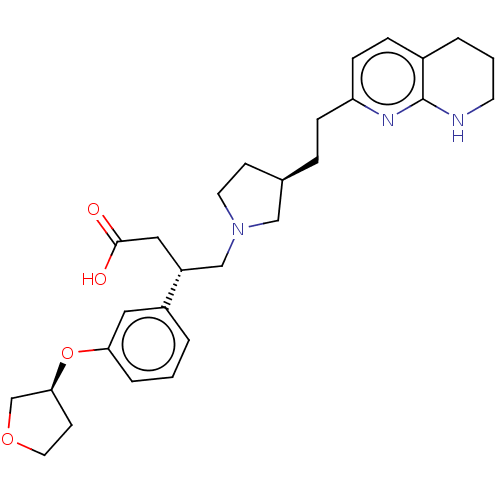 (CHEMBL4249172) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464124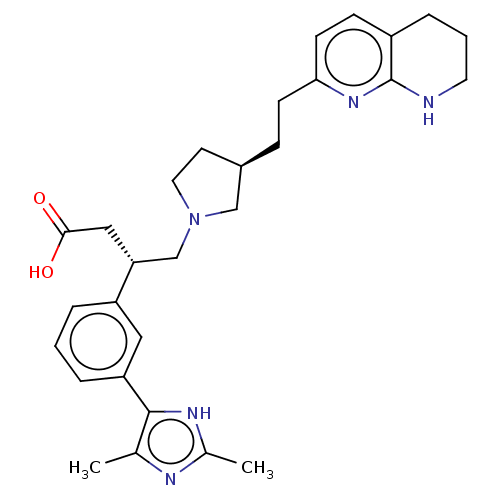 (CHEMBL4249629) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464108 (CHEMBL4241824) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384822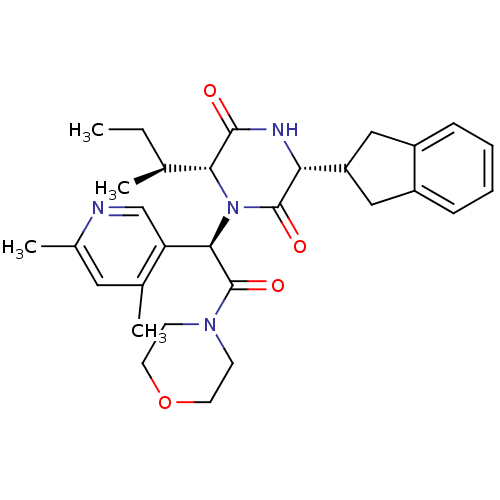 (CHEMBL2037508) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384818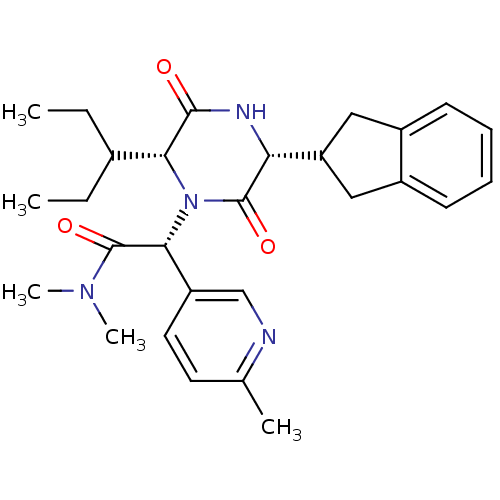 (CHEMBL2037513) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384837 (CHEMBL2037515) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464120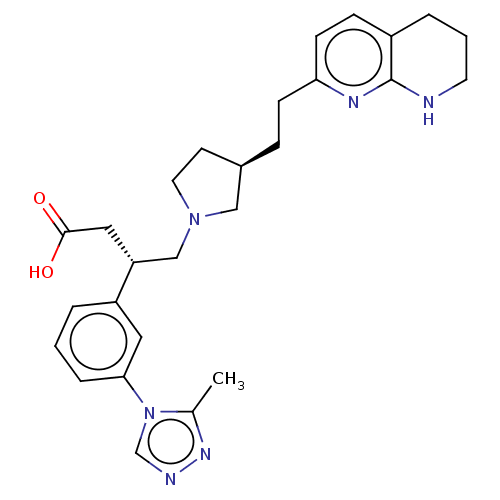 (CHEMBL4237919) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464097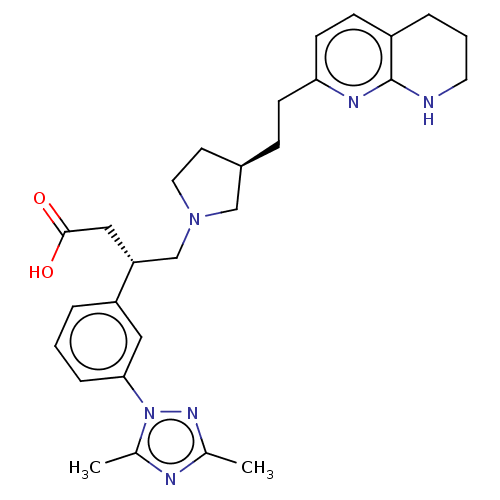 (CHEMBL4237868) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464113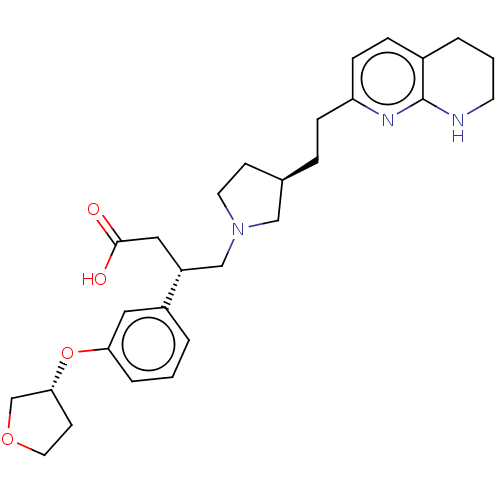 (CHEMBL4239085) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464110 (CHEMBL4238909) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464100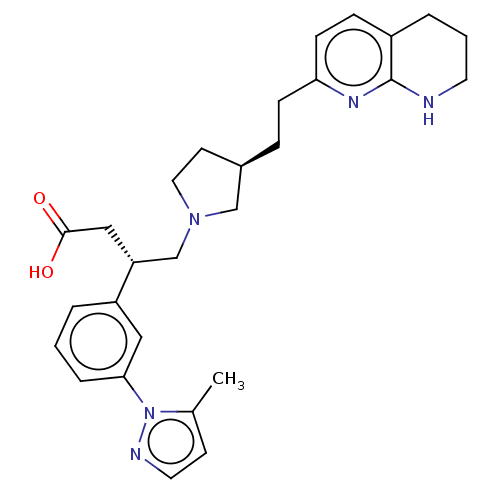 (CHEMBL4243367) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384824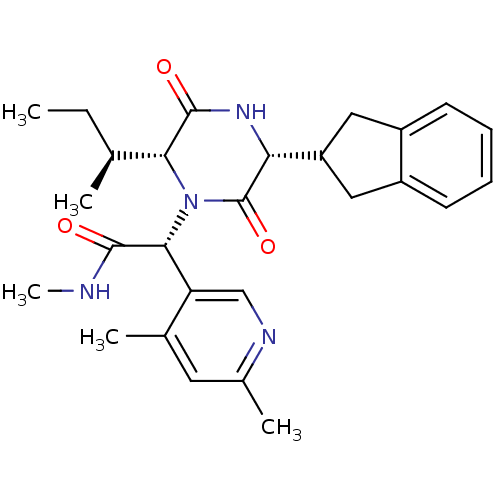 (CHEMBL2037506) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384815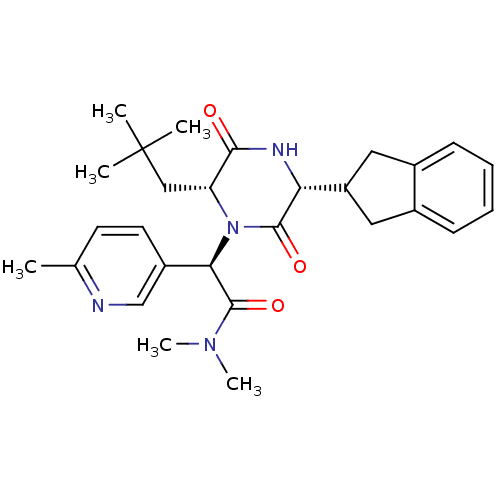 (CHEMBL2037496) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384834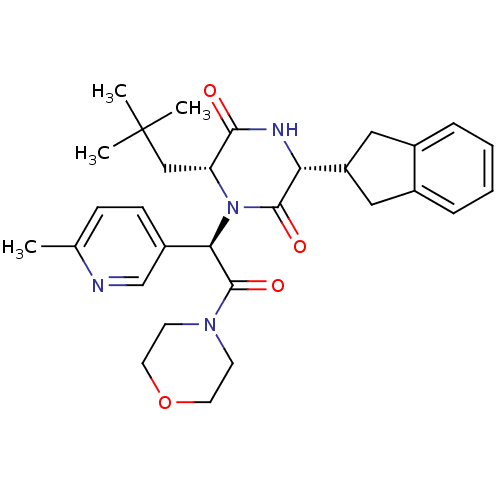 (CHEMBL2037497) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384838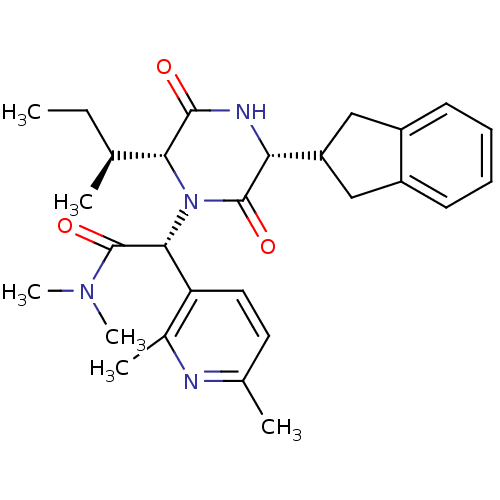 (CHEMBL2037510) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50190528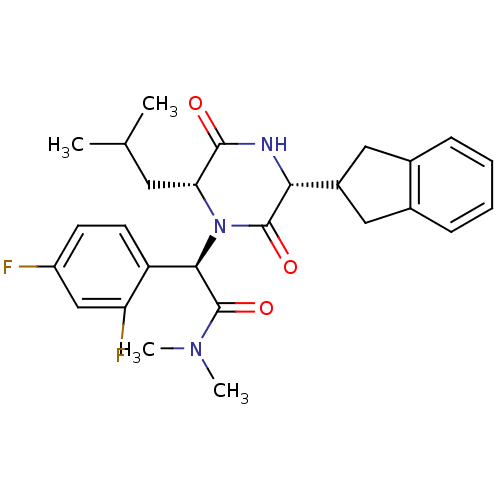 ((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464098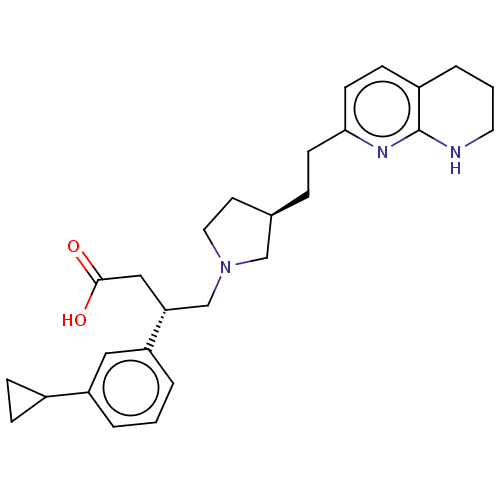 (CHEMBL4242635) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464115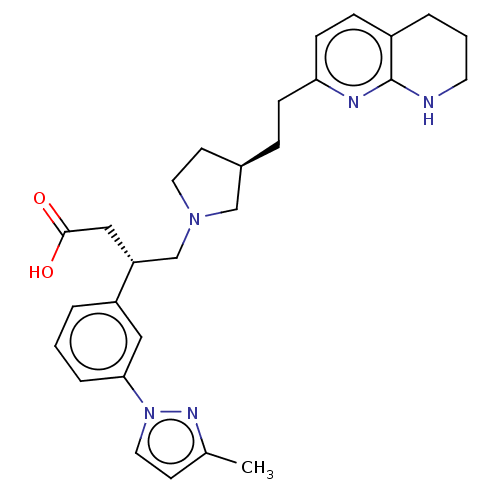 (CHEMBL4247149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464112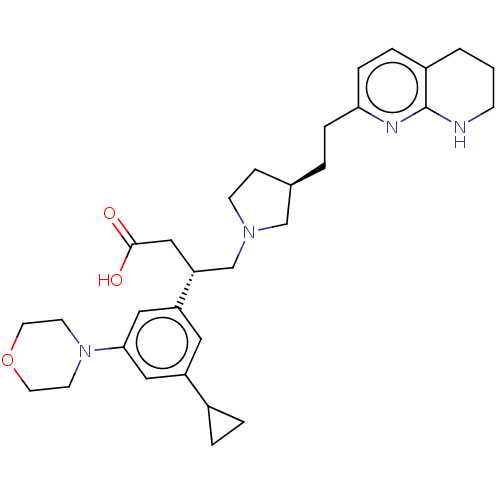 (CHEMBL4248589) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464102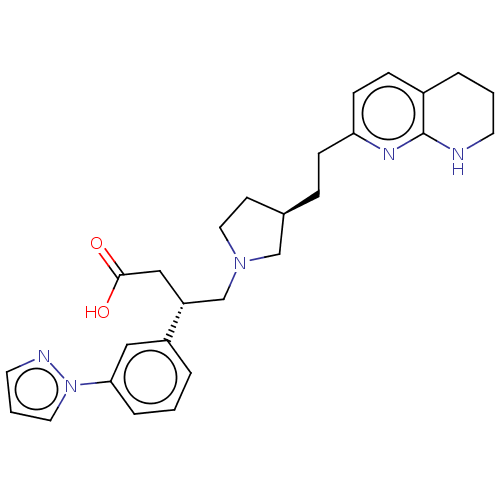 (CHEMBL4251444) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418268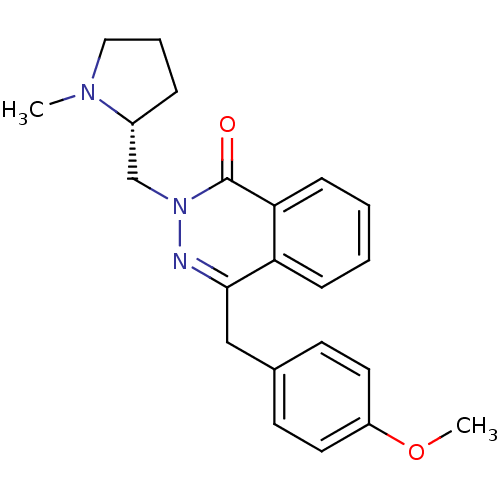 (CHEMBL1767137) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384819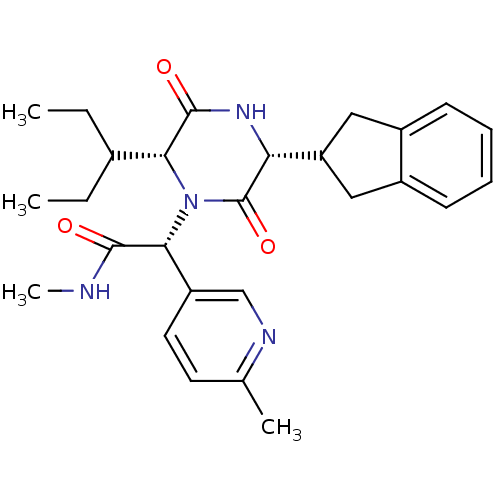 (CHEMBL2037512) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384820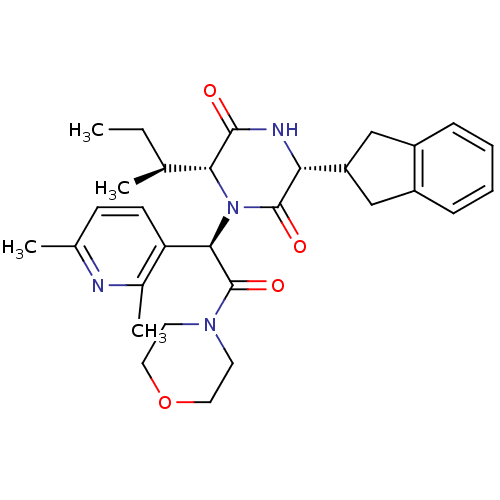 (EPELSIBAN | GSK557296B) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384803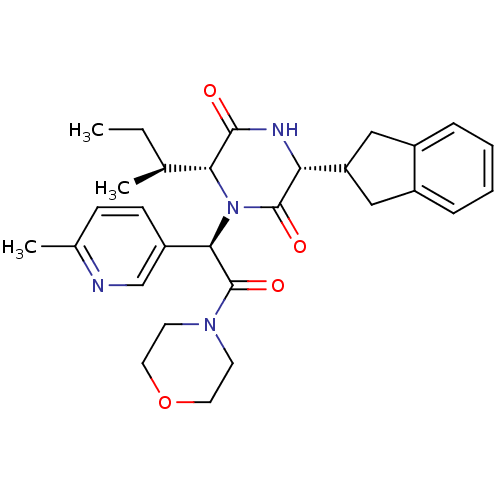 (CHEMBL2037501) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391708 (CHEMBL1767136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418267 (CHEMBL1767138) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391708 (CHEMBL1767136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418266 (CHEMBL1767141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384805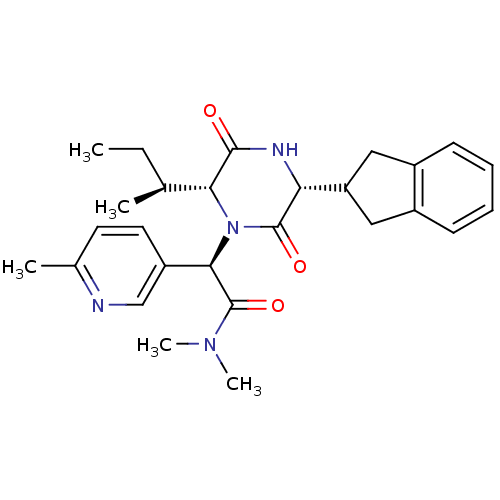 (CHEMBL2037499) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418298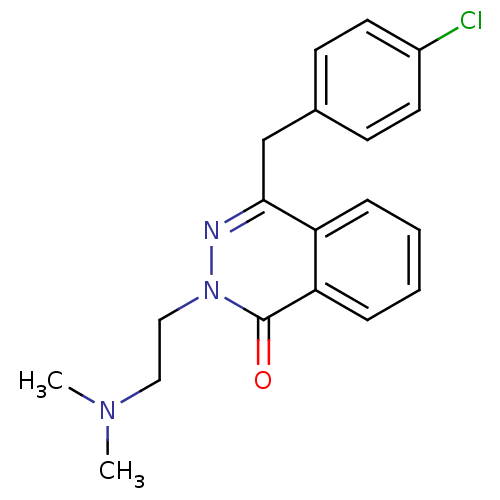 (CHEMBL1767134) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384812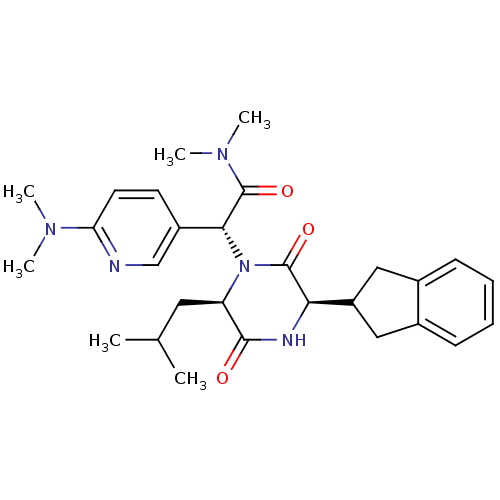 (CHEMBL2037489) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391698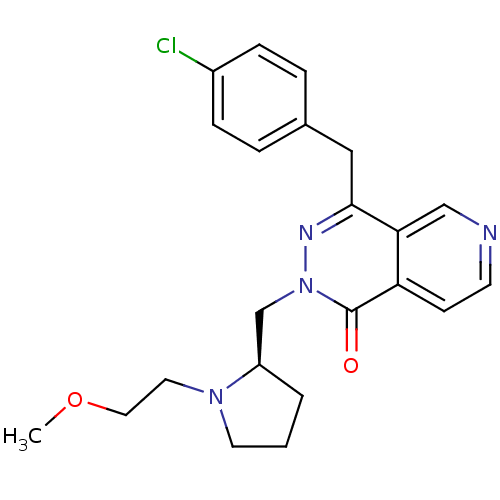 (CHEMBL2146801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384836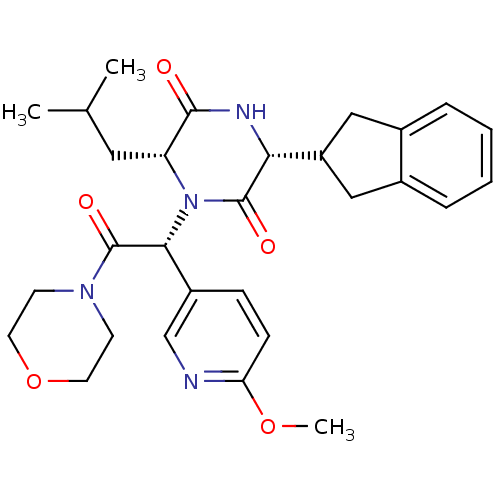 (CHEMBL2037487) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50341447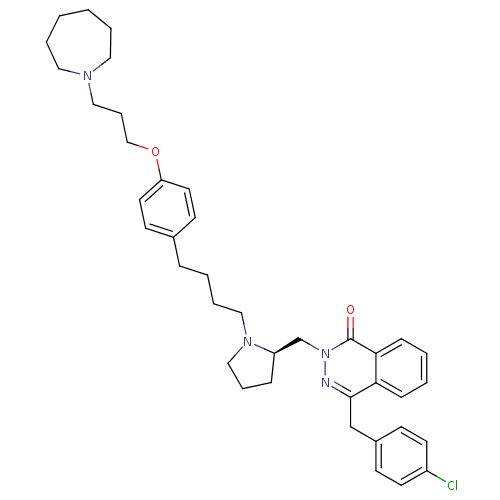 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox... | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418265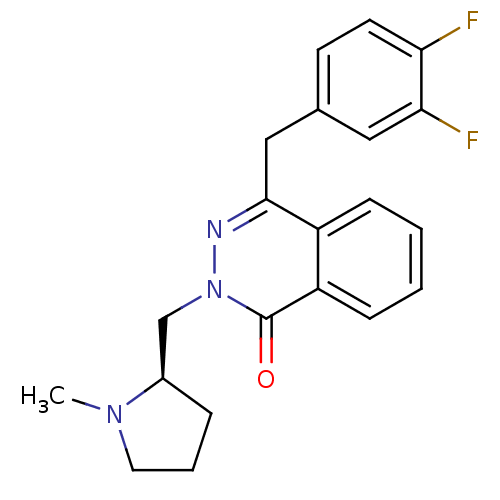 (CHEMBL1767149) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391702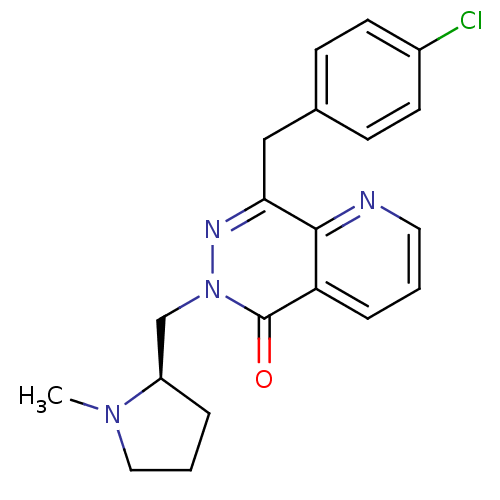 (CHEMBL2146809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418297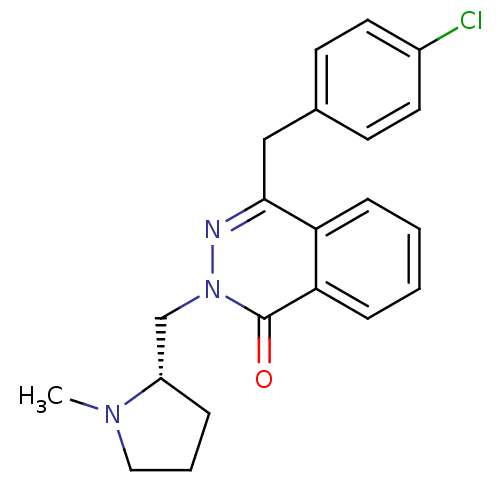 (CHEMBL1767154) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50418296 (CHEMBL1767140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... | J Med Chem 54: 2183-95 (2011) Article DOI: 10.1021/jm1013874 BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50384811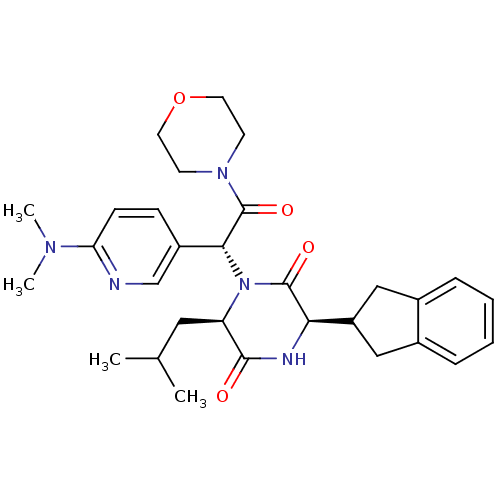 (CHEMBL2037490) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor | J Med Chem 55: 783-96 (2012) Article DOI: 10.1021/jm201287w BindingDB Entry DOI: 10.7270/Q2MS3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391707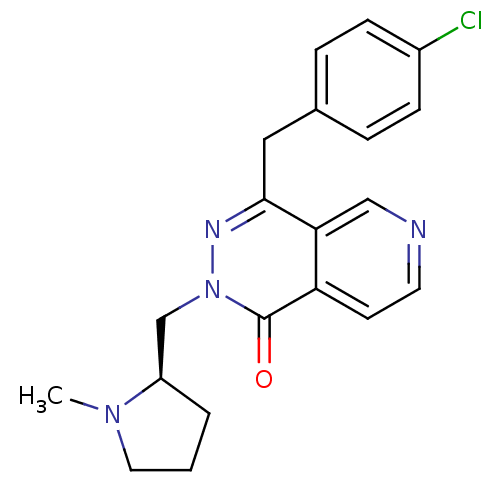 (CHEMBL2146805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391699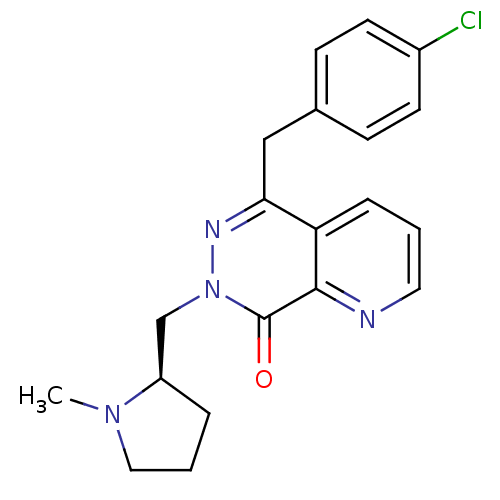 (CHEMBL2146484) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 755 total ) | Next | Last >> |