 Found 307 hits with Last Name = 'spacciapoli' and Initial = 'p'
Found 307 hits with Last Name = 'spacciapoli' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM50437406
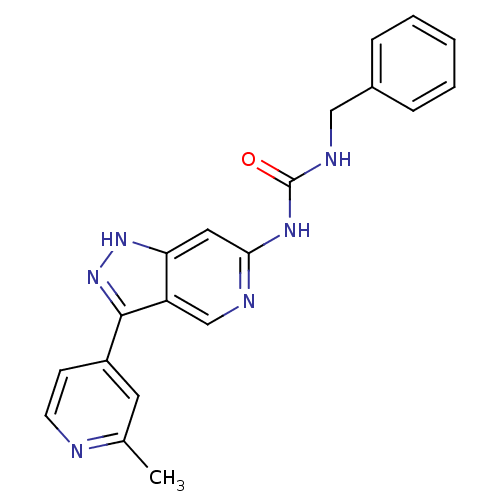
(CHEMBL2408788 | US9023865, 9)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCc3ccccc3)ncc12 Show InChI InChI=1S/C20H18N6O/c1-13-9-15(7-8-21-13)19-16-12-22-18(10-17(16)25-26-19)24-20(27)23-11-14-5-3-2-4-6-14/h2-10,12H,11H2,1H3,(H,25,26)(H2,22,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM50437406

(CHEMBL2408788 | US9023865, 9)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCc3ccccc3)ncc12 Show InChI InChI=1S/C20H18N6O/c1-13-9-15(7-8-21-13)19-16-12-22-18(10-17(16)25-26-19)24-20(27)23-11-14-5-3-2-4-6-14/h2-10,12H,11H2,1H3,(H,25,26)(H2,22,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50437407

(CHEMBL2408789 | US9023865, 6)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(C)c3)c2cn1)c1ccccc1 |r| Show InChI InChI=1S/C21H20N6O/c1-13-10-16(8-9-22-13)20-17-12-23-19(11-18(17)26-27-20)25-21(28)24-14(2)15-6-4-3-5-7-15/h3-12,14H,1-2H3,(H,26,27)(H2,23,24,25,28)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50437407

(CHEMBL2408789 | US9023865, 6)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(C)c3)c2cn1)c1ccccc1 |r| Show InChI InChI=1S/C21H20N6O/c1-13-10-16(8-9-22-13)20-17-12-23-19(11-18(17)26-27-20)25-21(28)24-14(2)15-6-4-3-5-7-15/h3-12,14H,1-2H3,(H,26,27)(H2,23,24,25,28)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157795

(US9023865, 636)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(F)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157185
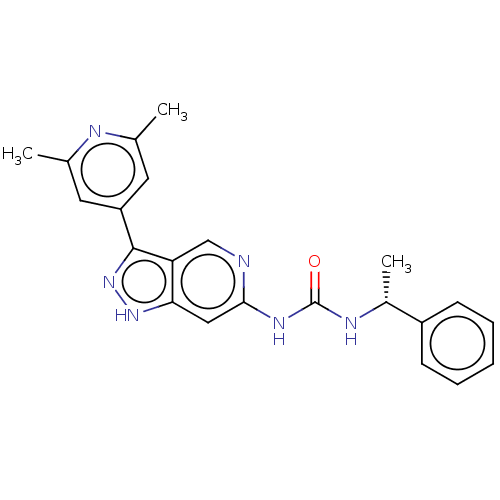
(US9023865, 31)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cc(C)nc(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157183

(US9023865, 29)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cnn(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157183

(US9023865, 29)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cnn(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157795

(US9023865, 636)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(F)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157185

(US9023865, 31)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cc(C)nc(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM157161
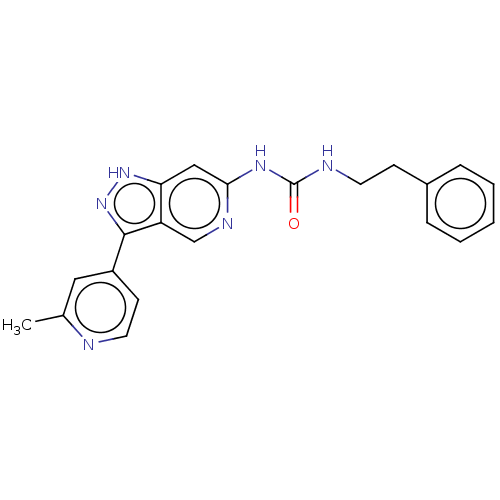
(US9023865, 5)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCCc3ccccc3)ncc12 Show InChI InChI=1S/C21H20N6O/c1-14-11-16(8-10-22-14)20-17-13-24-19(12-18(17)26-27-20)25-21(28)23-9-7-15-5-3-2-4-6-15/h2-6,8,10-13H,7,9H2,1H3,(H,26,27)(H2,23,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM157161

(US9023865, 5)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCCc3ccccc3)ncc12 Show InChI InChI=1S/C21H20N6O/c1-14-11-16(8-10-22-14)20-17-13-24-19(12-18(17)26-27-20)25-21(28)23-9-7-15-5-3-2-4-6-15/h2-6,8,10-13H,7,9H2,1H3,(H,26,27)(H2,23,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157184
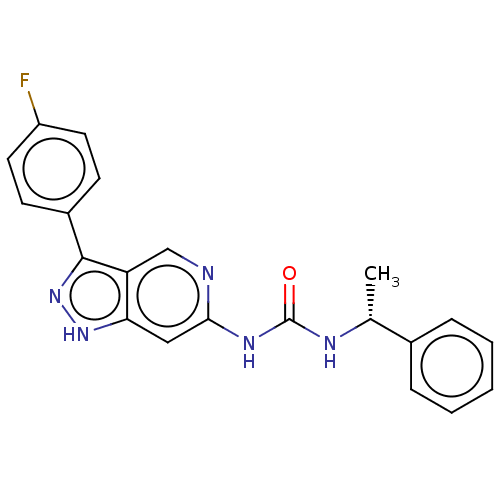
(US9023865, 30)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccc(F)cc3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552941

(CHEMBL4764710)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157184

(US9023865, 30)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccc(F)cc3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157820

(US9023865, 661)Show SMILES COc1cc(ccn1)-c1n[nH]c2cc(NC(=O)N[C@H](C)c3ccccc3)ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50437406

(CHEMBL2408788 | US9023865, 9)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCc3ccccc3)ncc12 Show InChI InChI=1S/C20H18N6O/c1-13-9-15(7-8-21-13)19-16-12-22-18(10-17(16)25-26-19)24-20(27)23-11-14-5-3-2-4-6-14/h2-10,12H,11H2,1H3,(H,25,26)(H2,22,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50437406

(CHEMBL2408788 | US9023865, 9)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCc3ccccc3)ncc12 Show InChI InChI=1S/C20H18N6O/c1-13-9-15(7-8-21-13)19-16-12-22-18(10-17(16)25-26-19)24-20(27)23-11-14-5-3-2-4-6-14/h2-10,12H,11H2,1H3,(H,25,26)(H2,22,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157245
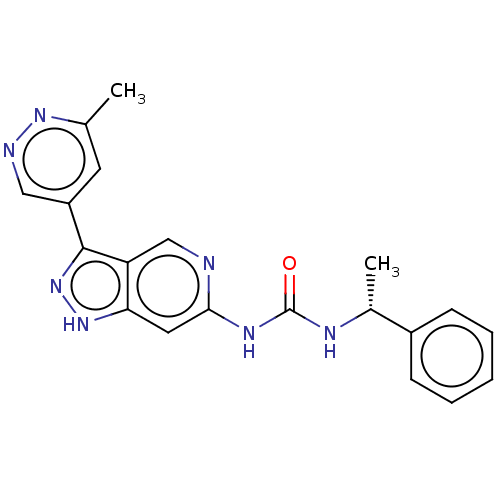
(US9023865, 91)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cnnc(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157820

(US9023865, 661)Show SMILES COc1cc(ccn1)-c1n[nH]c2cc(NC(=O)N[C@H](C)c3ccccc3)ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157245

(US9023865, 91)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cnnc(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561042
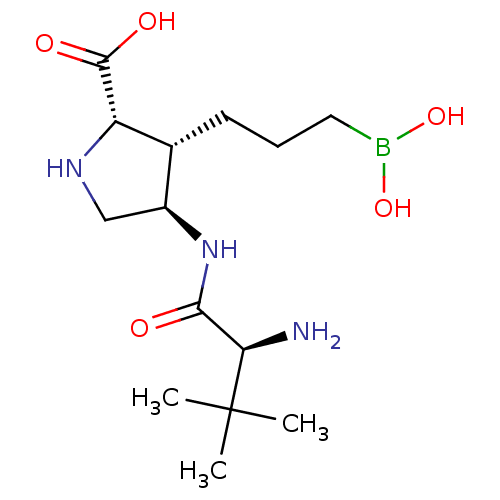
(CHEMBL4748950)Show SMILES CC(C)(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552934

(CHEMBL4744727)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552931

(CHEMBL4755227)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579614

(CHEMBL4871970)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)CCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552948

(CHEMBL4779920)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccncn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538499
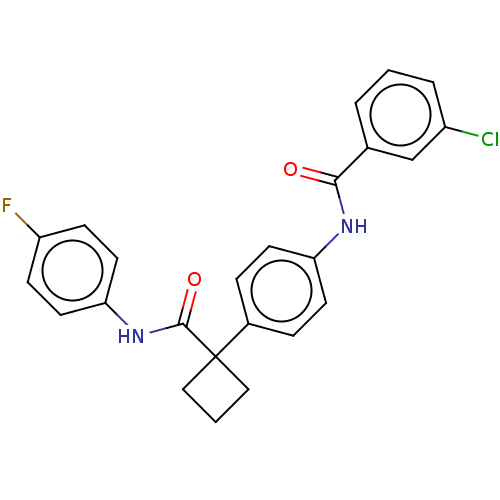
(CHEMBL4638223)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C24H20ClFN2O2/c25-18-4-1-3-16(15-18)22(29)27-20-9-5-17(6-10-20)24(13-2-14-24)23(30)28-21-11-7-19(26)8-12-21/h1,3-12,15H,2,13-14H2,(H,27,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of heme binding to IDO1 in interferon-gamma-induced human HeLa cells assessed as reduction in N-formylkynurenine production using L-trypto... |
ACS Med Chem Lett 11: 550-557 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00010
BindingDB Entry DOI: 10.7270/Q2FJ2M9D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434486

(CHEMBL2385548)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C34H28N6O2/c35-18-23-5-7-24(8-6-23)32-17-27-15-26-16-29(39-13-11-36-12-14-39)9-10-31(26)40(34(41)33(27)42-32)21-25-3-1-2-4-30(25)28-19-37-22-38-20-28/h1-10,16-17,19-20,22,36H,11-15,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157176
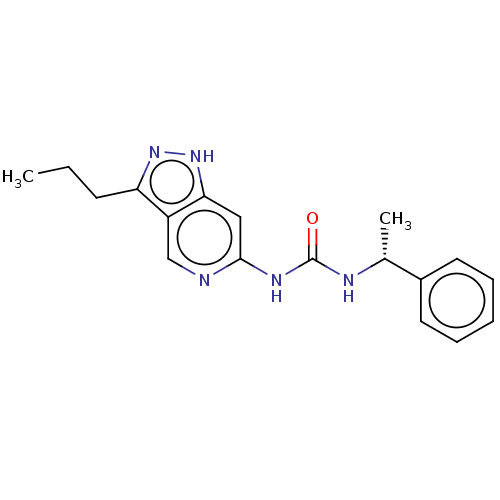
(US9023865, 22)Show SMILES CCCc1n[nH]c2cc(NC(=O)N[C@H](C)c3ccccc3)ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157179
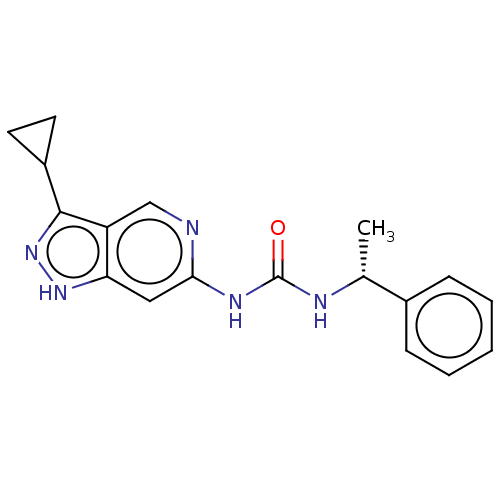
(US9023865, 25)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(C3CC3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157176

(US9023865, 22)Show SMILES CCCc1n[nH]c2cc(NC(=O)N[C@H](C)c3ccccc3)ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577469
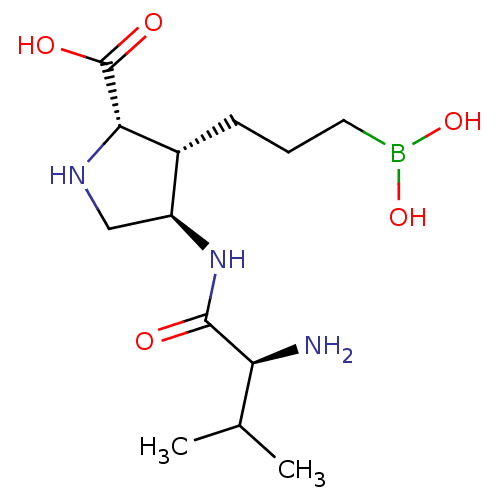
(CHEMBL4850213)Show SMILES CC(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157179

(US9023865, 25)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(C3CC3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
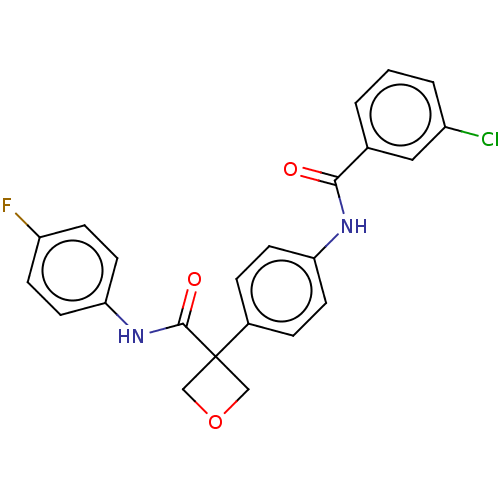
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of heme binding to IDO1 in interferon-gamma-induced human HeLa cells assessed as reduction in N-formylkynurenine production using L-trypto... |
ACS Med Chem Lett 11: 550-557 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00010
BindingDB Entry DOI: 10.7270/Q2FJ2M9D |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579610

(CHEMBL4873520)Show SMILES [H][C@]12CN[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:9:8:7.6.5:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552940

(CHEMBL4749009)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538505
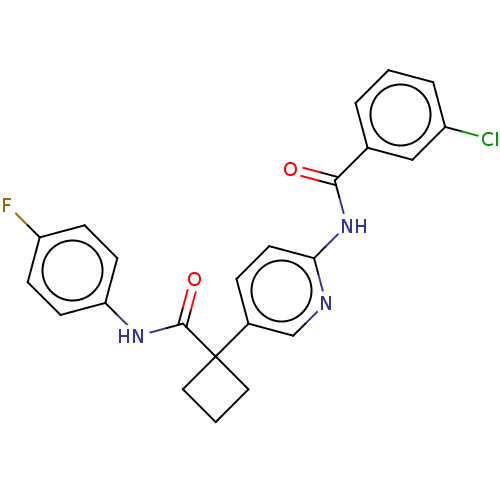
(CHEMBL4635112)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc(NC(=O)c3cccc(Cl)c3)nc2)cc1 Show InChI InChI=1S/C23H19ClFN3O2/c24-17-4-1-3-15(13-17)21(29)28-20-10-5-16(14-26-20)23(11-2-12-23)22(30)27-19-8-6-18(25)7-9-19/h1,3-10,13-14H,2,11-12H2,(H,27,30)(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of heme binding to IDO1 in interferon-gamma-induced human HeLa cells assessed as reduction in N-formylkynurenine production using L-trypto... |
ACS Med Chem Lett 11: 550-557 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00010
BindingDB Entry DOI: 10.7270/Q2FJ2M9D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552930
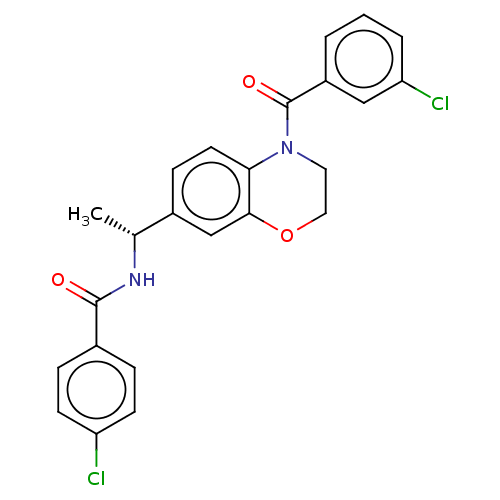
(CHEMBL4744926)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCOc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434488
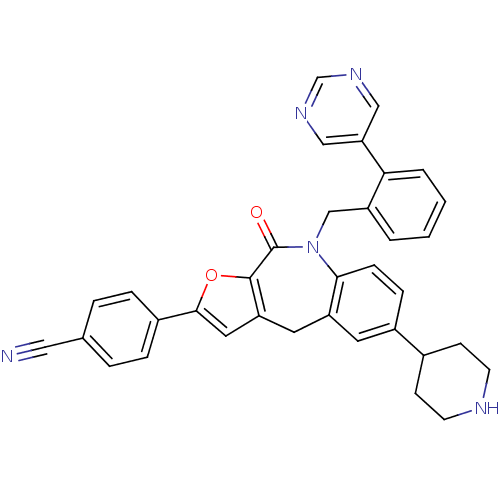
(CHEMBL2385549)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)C1CCNCC1 Show InChI InChI=1S/C35H29N5O2/c36-18-23-5-7-25(8-6-23)33-17-29-16-28-15-26(24-11-13-37-14-12-24)9-10-32(28)40(35(41)34(29)42-33)21-27-3-1-2-4-31(27)30-19-38-22-39-20-30/h1-10,15,17,19-20,22,24,37H,11-14,16,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552937

(CHEMBL4788473)Show SMILES CCOC(=O)N1CCCc2nc(ccc12)[C@@H](C)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561035
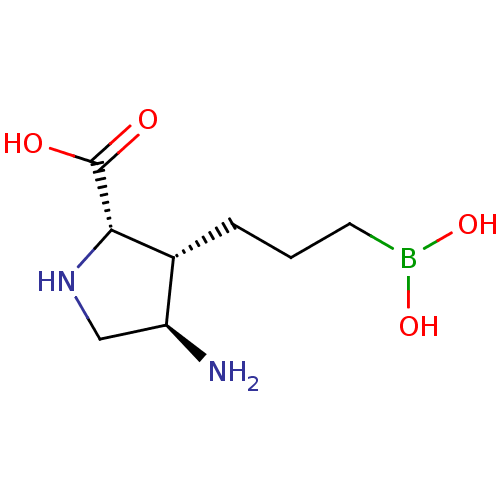
(CHEMBL4763578) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538501
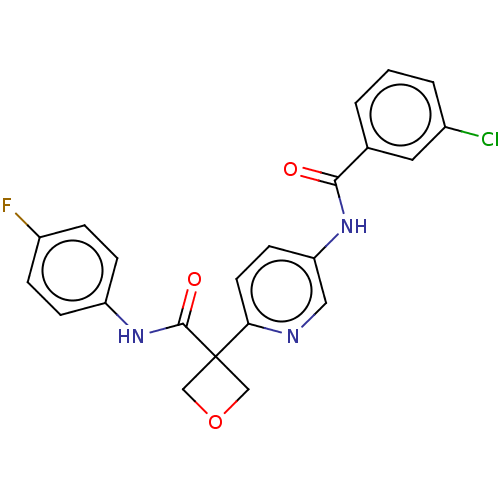
(CHEMBL4632601)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cn2)cc1 Show InChI InChI=1S/C22H17ClFN3O3/c23-15-3-1-2-14(10-15)20(28)26-18-8-9-19(25-11-18)22(12-30-13-22)21(29)27-17-6-4-16(24)5-7-17/h1-11H,12-13H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of heme binding to IDO1 in interferon-gamma-induced human HeLa cells assessed as reduction in N-formylkynurenine production using L-trypto... |
ACS Med Chem Lett 11: 550-557 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00010
BindingDB Entry DOI: 10.7270/Q2FJ2M9D |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579616

(CHEMBL4845730)Show SMILES [H][C@]12CN[C@H](C(=O)OC)[C@@]1(CCCB(O)O)CCCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538497
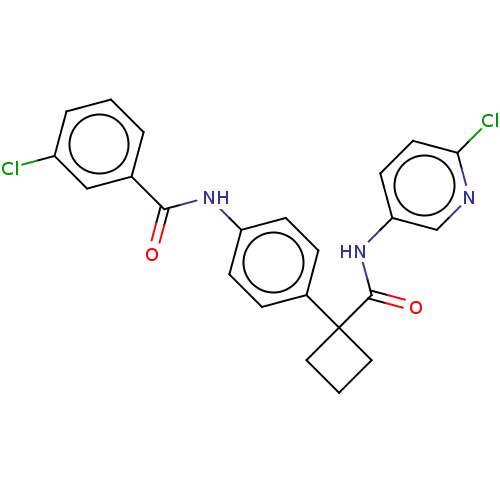
(CHEMBL4633874)Show SMILES Clc1cccc(c1)C(=O)Nc1ccc(cc1)C1(CCC1)C(=O)Nc1ccc(Cl)nc1 Show InChI InChI=1S/C23H19Cl2N3O2/c24-17-4-1-3-15(13-17)21(29)27-18-7-5-16(6-8-18)23(11-2-12-23)22(30)28-19-9-10-20(25)26-14-19/h1,3-10,13-14H,2,11-12H2,(H,27,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of heme binding to IDO1 in interferon-gamma-induced human HeLa cells assessed as reduction in N-formylkynurenine production using L-trypto... |
ACS Med Chem Lett 11: 550-557 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00010
BindingDB Entry DOI: 10.7270/Q2FJ2M9D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552949

(CHEMBL4779248)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579611
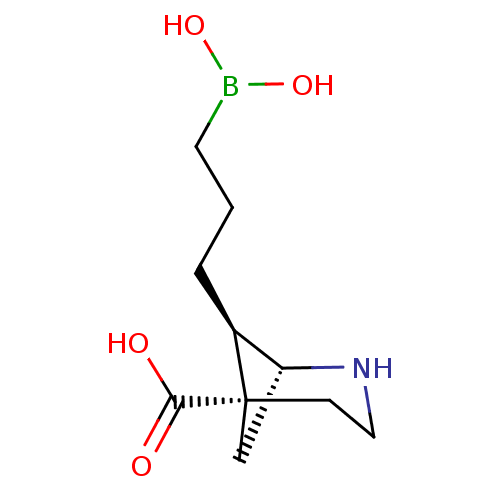
(CHEMBL4871097)Show SMILES [H][C@]12C[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:8:7:2:4.5.6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561036
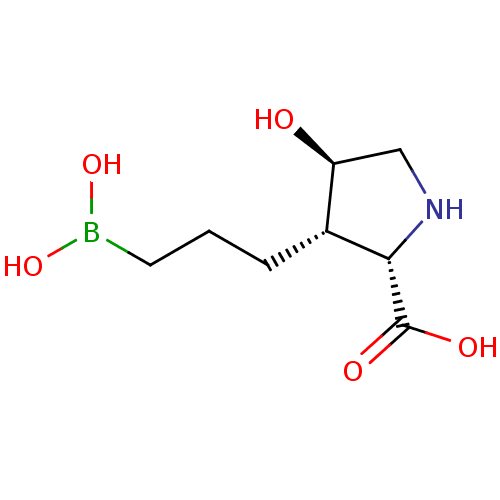
(CHEMBL4778978)Show SMILES OB(O)CCC[C@@H]1[C@@H](O)CN[C@@H]1C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data