

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50323701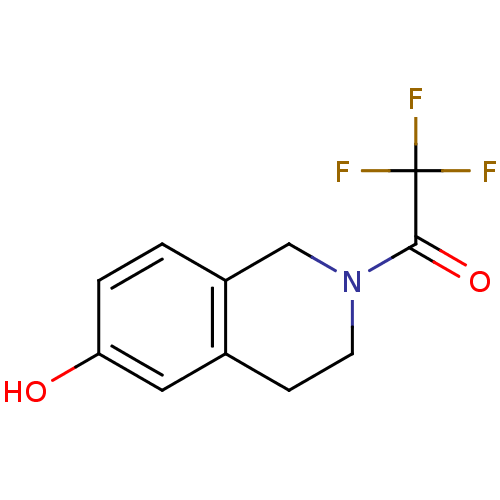 (2-(Trifluoroacetyl)-1,2,3,4-tetrahydro-6-isoquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human ERalpha ligand binding domain expressed in Escherichia coli BL21 (DE3) | Nat Chem Biol 5: 585-92 (2009) Article DOI: 10.1038/nchembio.188 BindingDB Entry DOI: 10.7270/Q2MP53F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50116538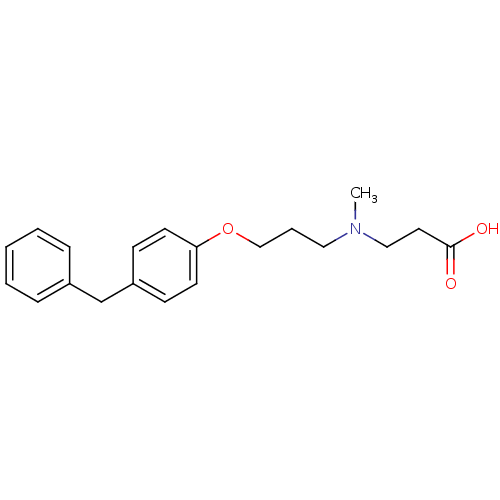 (3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Non-competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50197085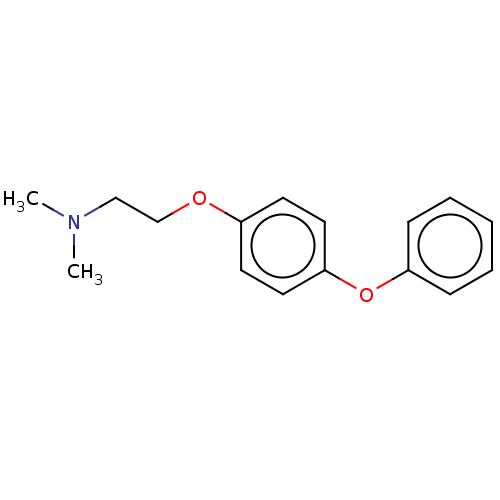 (CHEMBL3921982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Binding affinity to beta1 adrenergic receptor (unknown origin) by radioligand binding assay | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50197084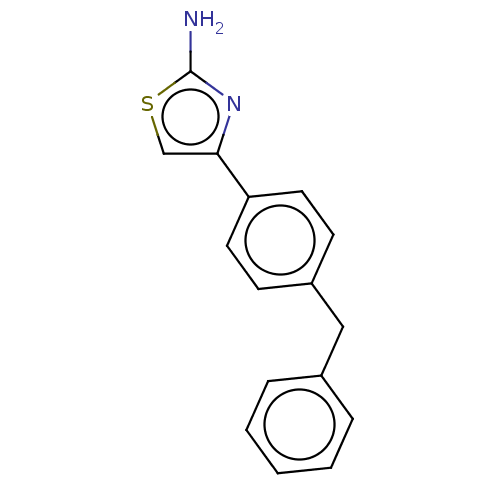 (CHEMBL3883608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Binding affinity to beta2 adrenergic receptor (unknown origin) by radioligand binding assay | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50530480 (CHEMBL4445524) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... | J Med Chem 62: 8443-8460 (2019) Article DOI: 10.1021/acs.jmedchem.9b00445 BindingDB Entry DOI: 10.7270/Q2V98CJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50530480 (CHEMBL4445524) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... | J Med Chem 62: 8443-8460 (2019) Article DOI: 10.1021/acs.jmedchem.9b00445 BindingDB Entry DOI: 10.7270/Q2V98CJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50264106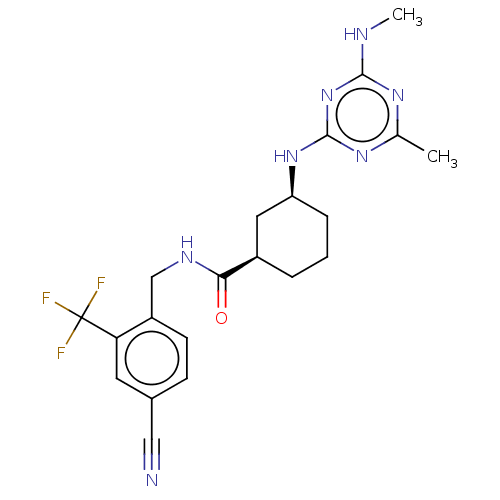 (CHEMBL3818875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50241116 (CHEMBL4066332) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of sEH in human HepG2 cells using 14(15)-EET-d11 as substrate assessed as reduction in conversion of 14(15)-EET-d11 to 14(15)-DHET-d11 pre... | J Med Chem 60: 7703-7724 (2017) Article DOI: 10.1021/acs.jmedchem.7b00398 BindingDB Entry DOI: 10.7270/Q2N3003W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561492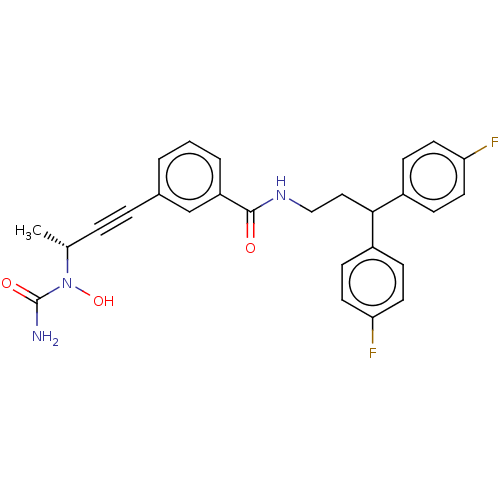 (CHEMBL4800490) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561490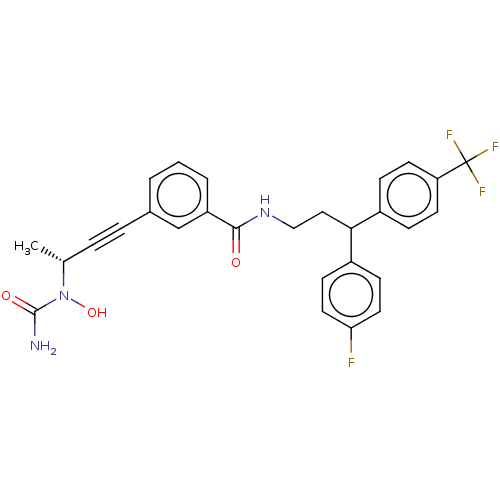 (CHEMBL4746942) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586956 (CHEMBL5075652) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586961 (CHEMBL5078180) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586964 (CHEMBL5074984) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586969 (CHEMBL5088299) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561482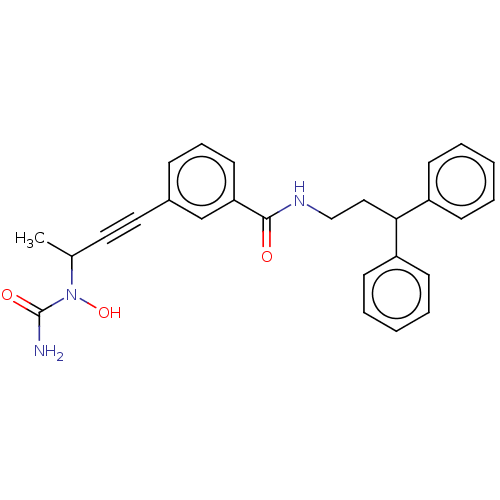 (CHEMBL4764099) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561483 (CHEMBL4795110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561486 (CHEMBL4759111) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561489 (CHEMBL4745687) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561488 (CHEMBL4745452) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561491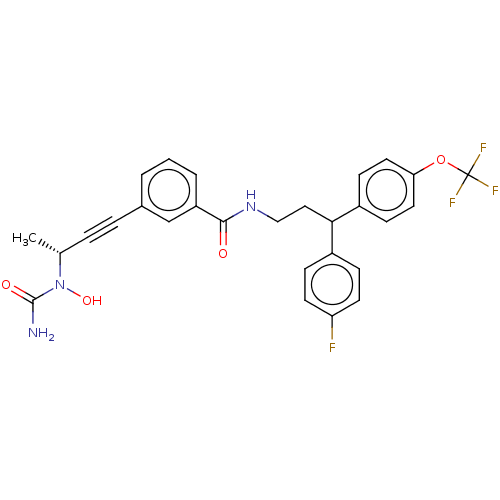 (CHEMBL4755533) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561484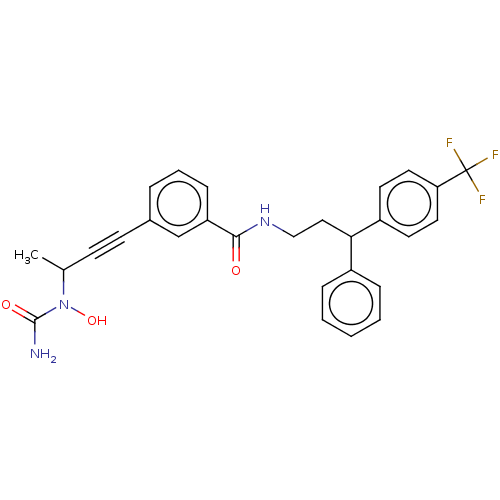 (CHEMBL4778283) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586945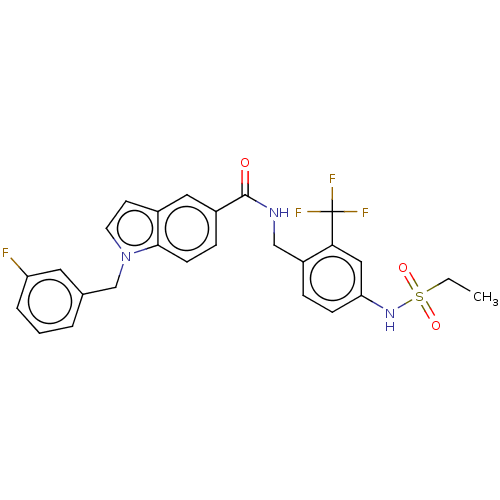 (CHEMBL5084314) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586954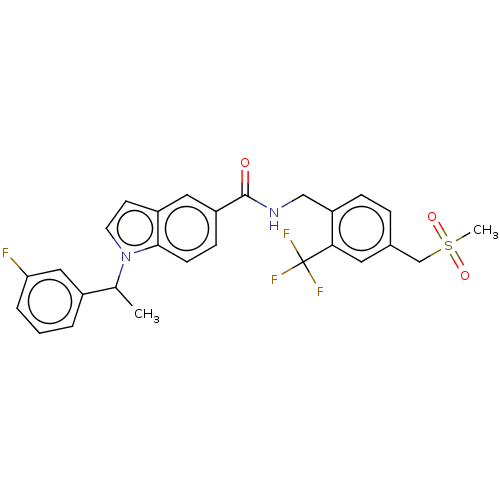 (CHEMBL5079563) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586962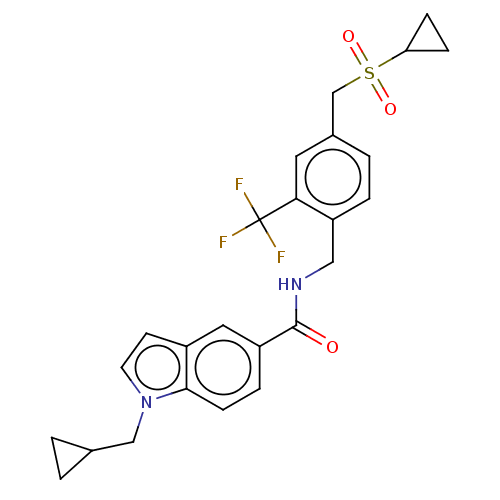 (CHEMBL5081457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561495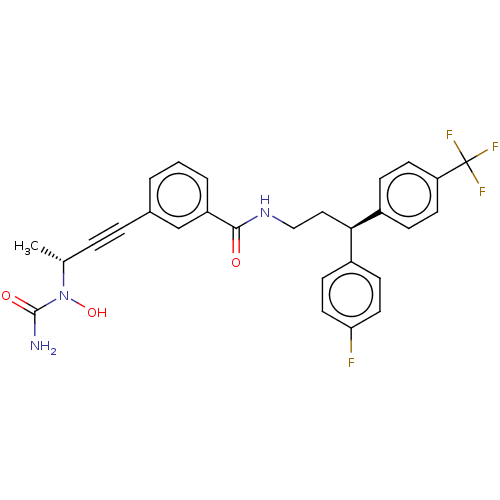 (CHEMBL4794423) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586965 (CHEMBL5074074) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561487 (CHEMBL4759652) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561494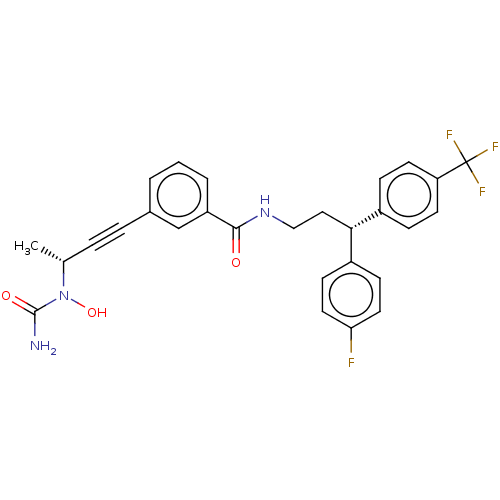 (CHEMBL4751593) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586963 (CHEMBL5090087) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586955 (CHEMBL5090873) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561480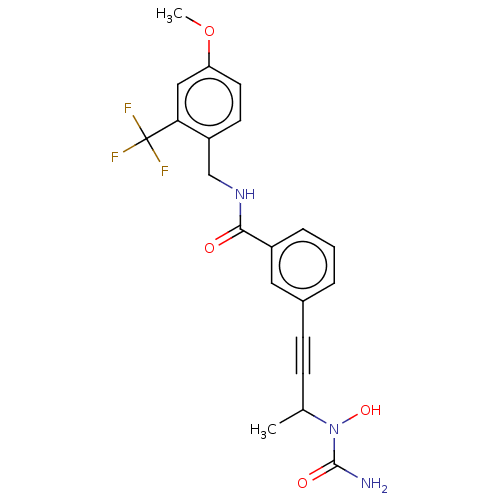 (CHEMBL4791222) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50241116 (CHEMBL4066332) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of recombinant v-Abl tyrosine kinase. | J Med Chem 60: 7703-7724 (2017) Article DOI: 10.1021/acs.jmedchem.7b00398 BindingDB Entry DOI: 10.7270/Q2N3003W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM13066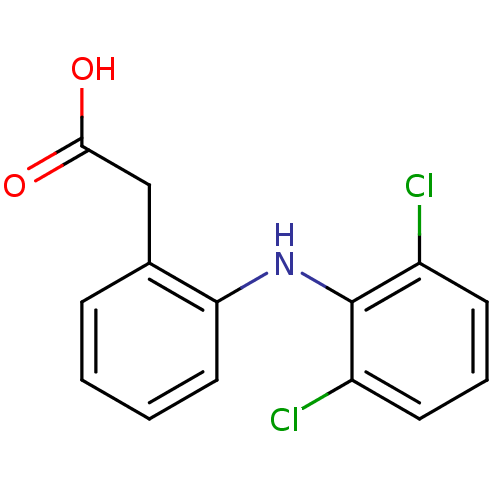 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586944 (CHEMBL5090821) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586947 (CHEMBL5087833) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561493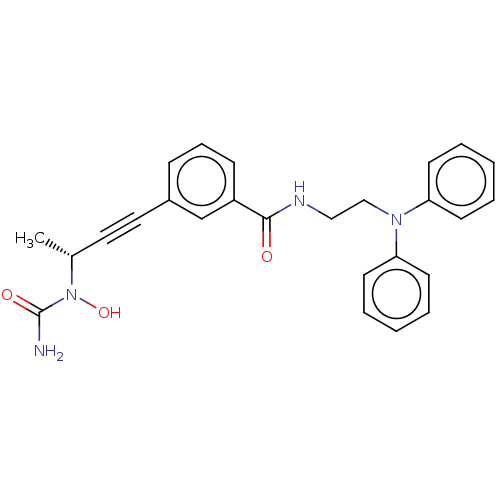 (CHEMBL4747688) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586966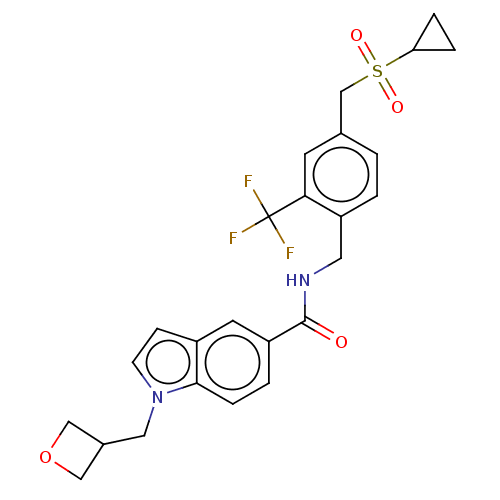 (CHEMBL5091134) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50561474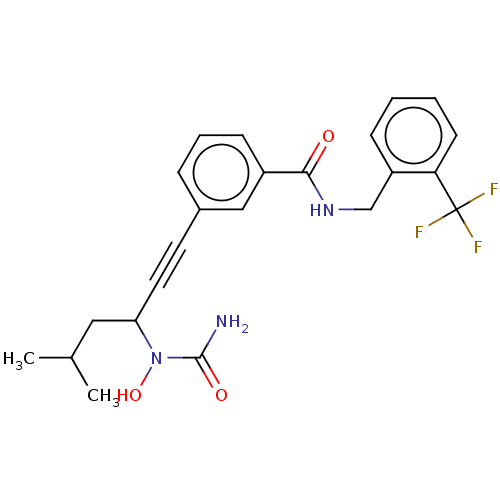 (CHEMBL4746544) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00561 BindingDB Entry DOI: 10.7270/Q2ST7TJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586960 (CHEMBL5081013) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586948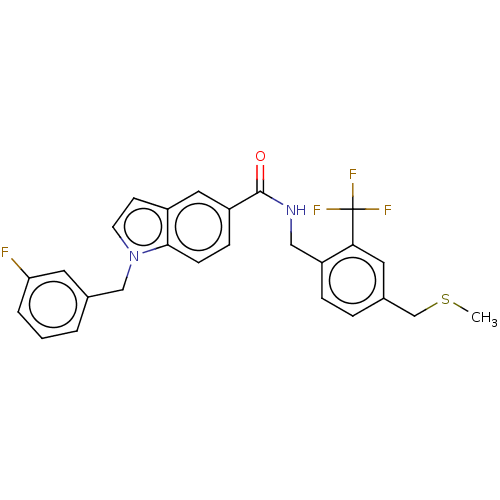 (CHEMBL5087046) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50116538 (3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50042235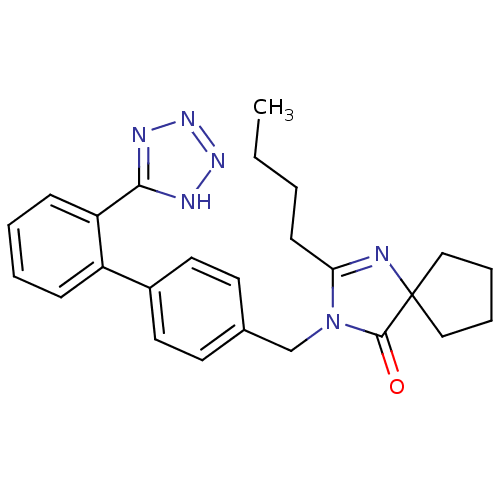 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human AT1 overexpressed in CHO-K1 cells in presence of 10 nM [Val5-angiotensin II measured after 90 mins by HTRF based IP-one ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00240 BindingDB Entry DOI: 10.7270/Q2FJ2MMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586949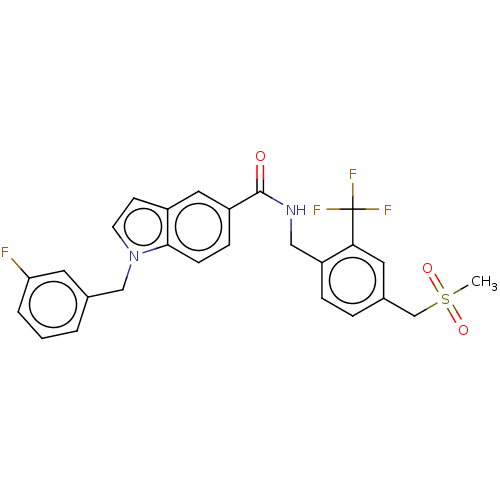 (CHEMBL5080474) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586952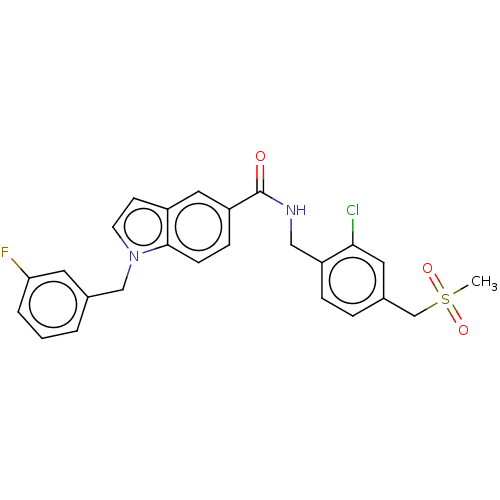 (CHEMBL5094953) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50586959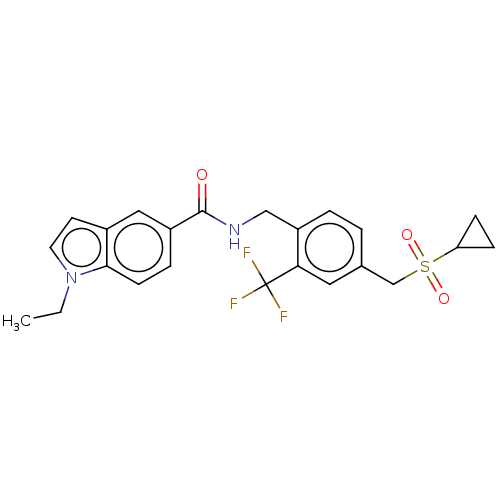 (CHEMBL5092964) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01331 BindingDB Entry DOI: 10.7270/Q2T43Z05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1271 total ) | Next | Last >> |