 Found 56 hits with Last Name = 'suenaga' and Initial = 'k'
Found 56 hits with Last Name = 'suenaga' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCeta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCtheta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCdelta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391388
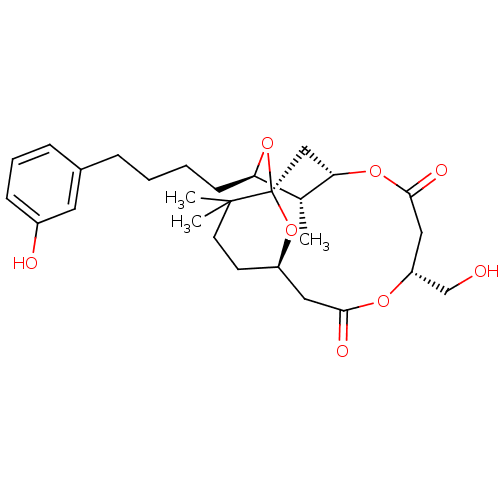
(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCgamma C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCalpha C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCepsilon C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCbeta C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391387
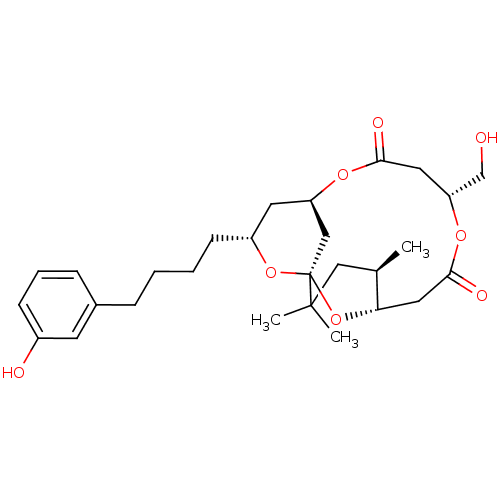
(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50327946
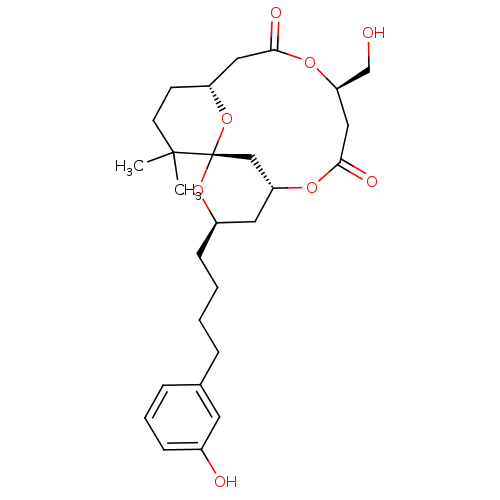
(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391388

(CHEMBL2148108)Show SMILES C[C@@H]1[C@@H](CCCCc2cccc(O)c2)O[C@]23C[C@@H]1OC(=O)C[C@H](CO)OC(=O)C[C@@H](CCC2(C)C)O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-23(10-5-4-7-19-8-6-9-20(30)13-19)36-28-16-24(18)34-26(32)15-22(17-29)33-25(31)14-21(35-28)11-12-27(28,2)3/h6,8-9,13,18,21-24,29-30H,4-5,7,10-12,14-17H2,1-3H3/t18-,21-,22-,23-,24?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391387

(CHEMBL2148107)Show SMILES C[C@@H]1CC(C)(C)[C@@]23C[C@@H](C[C@@H](CCCCc4cccc(O)c4)O2)OC(=O)C[C@H](CO)OC(=O)C[C@@H]1O3 |r| Show InChI InChI=1S/C28H40O8/c1-18-15-27(2,3)28-16-22(33-25(31)13-23(17-29)34-26(32)14-24(18)36-28)12-21(35-28)10-5-4-7-19-8-6-9-20(30)11-19/h6,8-9,11,18,21-24,29-30H,4-5,7,10,12-17H2,1-3H3/t18-,21-,22?,23-,24+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCepsilon C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCgamma C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50327946

(1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...)Show SMILES CC1(C)CC[C@@H]2CC(=O)O[C@@H](CO)CC(=O)O[C@@H]3C[C@@H](CCCCc4cccc(O)c4)O[C@@]1(C3)O2 |r| Show InChI InChI=1S/C27H38O8/c1-26(2)11-10-21-14-24(30)33-23(17-28)15-25(31)32-22-13-20(34-27(26,16-22)35-21)9-4-3-6-18-7-5-8-19(29)12-18/h5,7-8,12,20-23,28-29H,3-4,6,9-11,13-17H2,1-2H3/t20-,21-,22?,23-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 00.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D using GKPILFFRLK(DNP)-D-RNH2) labeled MCA as substrate preincubated for 10 mins followed by substrate addition measur... |
J Nat Prod 81: 1673-1681 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00417
BindingDB Entry DOI: 10.7270/Q2MW2KQC |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50361186
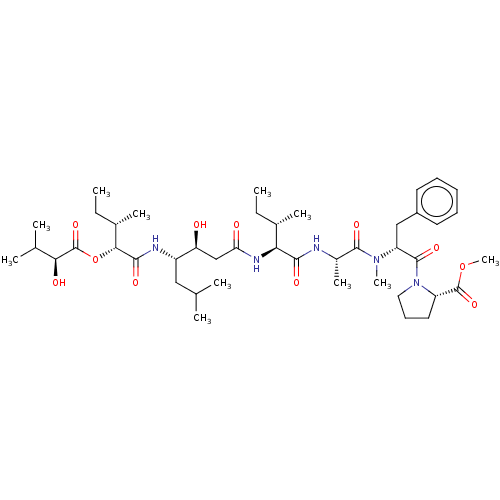
(CHEMBL4161264)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](O)C(C)C)[C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C44H71N5O11/c1-12-27(7)36(47-35(51)24-34(50)31(22-25(3)4)46-40(54)38(28(8)13-2)60-44(58)37(52)26(5)6)39(53)45-29(9)41(55)48(10)33(23-30-18-15-14-16-19-30)42(56)49-21-17-20-32(49)43(57)59-11/h14-16,18-19,25-29,31-34,36-38,50,52H,12-13,17,20-24H2,1-11H3,(H,45,53)(H,46,54)(H,47,51)/t27-,28-,29-,31-,32-,33+,34-,36-,37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D using GKPILFFRLK(DNP)-D-RNH2) labeled MCA as substrate preincubated for 10 mins followed by substrate addition measur... |
J Nat Prod 81: 1673-1681 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00417
BindingDB Entry DOI: 10.7270/Q2MW2KQC |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50361188
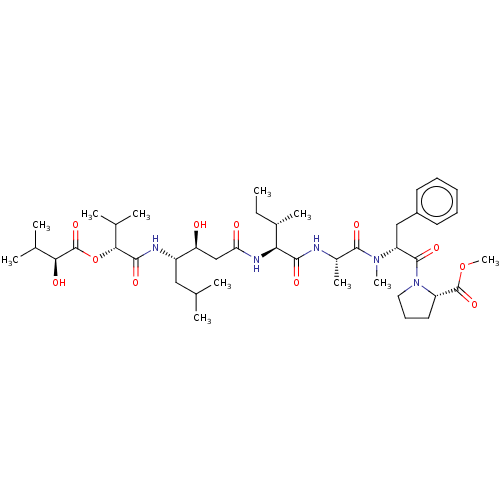
(CHEMBL4169189)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](O)C(C)C)C(C)C)C(=O)N[C@@H](C)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C43H69N5O11/c1-12-27(8)35(46-34(50)23-33(49)30(21-24(2)3)45-39(53)37(26(6)7)59-43(57)36(51)25(4)5)38(52)44-28(9)40(54)47(10)32(22-29-17-14-13-15-18-29)41(55)48-20-16-19-31(48)42(56)58-11/h13-15,17-18,24-28,30-33,35-37,49,51H,12,16,19-23H2,1-11H3,(H,44,52)(H,45,53)(H,46,50)/t27-,28-,30-,31-,32+,33-,35-,36-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D using GKPILFFRLK(DNP)-D-RNH2) labeled MCA as substrate preincubated for 10 mins followed by substrate addition measur... |
J Nat Prod 81: 1673-1681 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00417
BindingDB Entry DOI: 10.7270/Q2MW2KQC |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor ligand superfamily member 11
(Mus musculus) | BDBM50128235
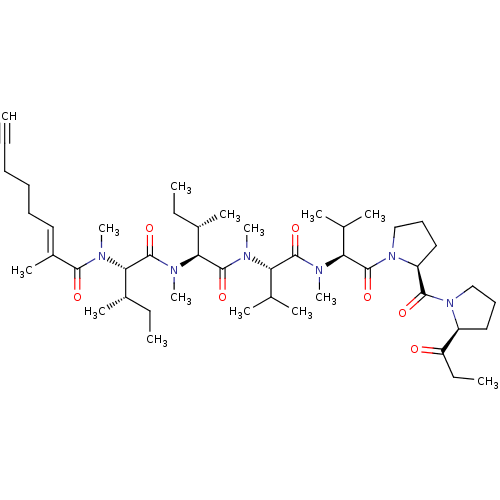
(CHEMBL3628709)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H]([C@@H](C)CC)N(C)C(=O)C(\C)=C\CCCC#C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)CC |r| Show InChI InChI=1S/C47H78N6O7/c1-16-20-21-22-25-34(11)42(55)50(14)40(32(9)17-2)46(59)51(15)41(33(10)18-3)45(58)48(12)38(30(5)6)44(57)49(13)39(31(7)8)47(60)53-29-24-27-36(53)43(56)52-28-23-26-35(52)37(54)19-4/h1,25,30-33,35-36,38-41H,17-24,26-29H2,2-15H3/b34-25+/t32-,33-,35-,36-,38-,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of M-CSF/sRANKL-induced mouse BMDM differentiation into osteoclast after 72 hrs by TRAP staining-based microscopic analysis |
Bioorg Med Chem Lett 25: 5295-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.044
BindingDB Entry DOI: 10.7270/Q2GX4DCP |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Chlorocebus aethiops) | BDBM50281511
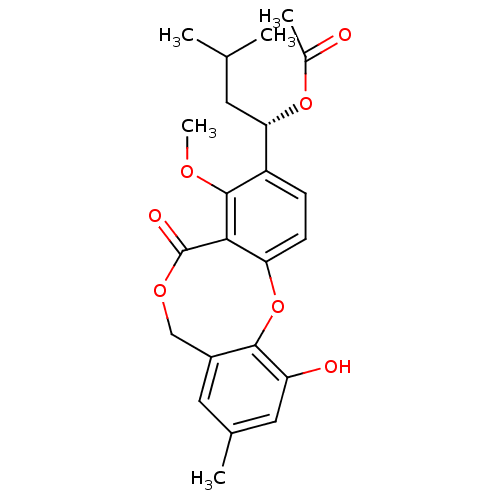
(Acetic acid (S)-1-(11-hydroxy-4-methoxy-9-methyl-5...)Show SMILES COc1c(ccc2Oc3c(O)cc(C)cc3COC(=O)c12)[C@H](CC(C)C)OC(C)=O Show InChI InChI=1S/C23H26O7/c1-12(2)8-19(29-14(4)24)16-6-7-18-20(22(16)27-5)23(26)28-11-15-9-13(3)10-17(25)21(15)30-18/h6-7,9-10,12,19,25H,8,11H2,1-5H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT1 expressed in CHO cell microsomal fractions assessed as reduction in neutral lipid synthesis incubated for 6 ... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Chlorocebus aethiops) | BDBM50450891
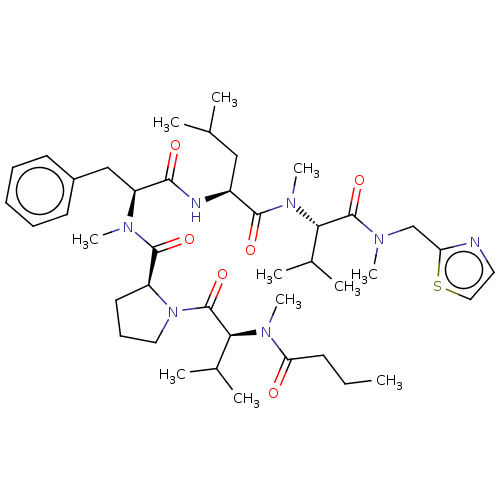
(CHEMBL4212280)Show SMILES CCCC(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C42H65N7O6S/c1-12-17-35(50)47(10)37(29(6)7)42(55)49-22-16-20-32(49)40(53)46(9)33(25-30-18-14-13-15-19-30)38(51)44-31(24-27(2)3)39(52)48(11)36(28(4)5)41(54)45(8)26-34-43-21-23-56-34/h13-15,18-19,21,23,27-29,31-33,36-37H,12,16-17,20,22,24-26H2,1-11H3,(H,44,51)/t31-,32-,33-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Chlorocebus aethiops) | BDBM50281511

(Acetic acid (S)-1-(11-hydroxy-4-methoxy-9-methyl-5...)Show SMILES COc1c(ccc2Oc3c(O)cc(C)cc3COC(=O)c12)[C@H](CC(C)C)OC(C)=O Show InChI InChI=1S/C23H26O7/c1-12(2)8-19(29-14(4)24)16-6-7-18-20(22(16)27-5)23(26)28-11-15-9-13(3)10-17(25)21(15)30-18/h6-7,9-10,12,19,25H,8,11H2,1-5H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Chlorocebus aethiops) | BDBM50450891

(CHEMBL4212280)Show SMILES CCCC(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C42H65N7O6S/c1-12-17-35(50)47(10)37(29(6)7)42(55)49-22-16-20-32(49)40(53)46(9)33(25-30-18-14-13-15-19-30)38(51)44-31(24-27(2)3)39(52)48(11)36(28(4)5)41(54)45(8)26-34-43-21-23-56-34/h13-15,18-19,21,23,27-29,31-33,36-37H,12,16-17,20,22,24-26H2,1-11H3,(H,44,51)/t31-,32-,33-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Chlorocebus aethiops) | BDBM50281511

(Acetic acid (S)-1-(11-hydroxy-4-methoxy-9-methyl-5...)Show SMILES COc1c(ccc2Oc3c(O)cc(C)cc3COC(=O)c12)[C@H](CC(C)C)OC(C)=O Show InChI InChI=1S/C23H26O7/c1-12(2)8-19(29-14(4)24)16-6-7-18-20(22(16)27-5)23(26)28-11-15-9-13(3)10-17(25)21(15)30-18/h6-7,9-10,12,19,25H,8,11H2,1-5H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT2 expressed in CHO cell microsomal fractions assessed as reduction in neutral lipid synthesis incubated for 6 ... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Chlorocebus aethiops) | BDBM50450891

(CHEMBL4212280)Show SMILES CCCC(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C42H65N7O6S/c1-12-17-35(50)47(10)37(29(6)7)42(55)49-22-16-20-32(49)40(53)46(9)33(25-30-18-14-13-15-19-30)38(51)44-31(24-27(2)3)39(52)48(11)36(28(4)5)41(54)45(8)26-34-43-21-23-56-34/h13-15,18-19,21,23,27-29,31-33,36-37H,12,16-17,20,22,24-26H2,1-11H3,(H,44,51)/t31-,32-,33-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT1 expressed in CHO cell microsomal fractions assessed as reduction in neutral lipid synthesis incubated for 6 ... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Chlorocebus aethiops) | BDBM50450893

(CHEMBL4207391)Show SMILES CCCC(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C41H63N7O6S/c1-11-16-34(49)47(10)36(28(6)7)41(54)48-21-15-19-31(48)39(52)46(9)32(24-29-17-13-12-14-18-29)38(51)43-30(23-26(2)3)37(50)44-35(27(4)5)40(53)45(8)25-33-42-20-22-55-33/h12-14,17-18,20,22,26-28,30-32,35-36H,11,15-16,19,21,23-25H2,1-10H3,(H,43,51)(H,44,50)/t30-,31-,32-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Chlorocebus aethiops) | BDBM50281511

(Acetic acid (S)-1-(11-hydroxy-4-methoxy-9-methyl-5...)Show SMILES COc1c(ccc2Oc3c(O)cc(C)cc3COC(=O)c12)[C@H](CC(C)C)OC(C)=O Show InChI InChI=1S/C23H26O7/c1-12(2)8-19(29-14(4)24)16-6-7-18-20(22(16)27-5)23(26)28-11-15-9-13(3)10-17(25)21(15)30-18/h6-7,9-10,12,19,25H,8,11H2,1-5H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Chlorocebus aethiops) | BDBM50450893

(CHEMBL4207391)Show SMILES CCCC(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C41H63N7O6S/c1-11-16-34(49)47(10)36(28(6)7)41(54)48-21-15-19-31(48)39(52)46(9)32(24-29-17-13-12-14-18-29)38(51)43-30(23-26(2)3)37(50)44-35(27(4)5)40(53)45(8)25-33-42-20-22-55-33/h12-14,17-18,20,22,26-28,30-32,35-36H,11,15-16,19,21,23-25H2,1-10H3,(H,43,51)(H,44,50)/t30-,31-,32-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT1 expressed in CHO cell microsomal fractions assessed as reduction in neutral lipid synthesis incubated for 6 ... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Chlorocebus aethiops) | BDBM50450893

(CHEMBL4207391)Show SMILES CCCC(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C41H63N7O6S/c1-11-16-34(49)47(10)36(28(6)7)41(54)48-21-15-19-31(48)39(52)46(9)32(24-29-17-13-12-14-18-29)38(51)43-30(23-26(2)3)37(50)44-35(27(4)5)40(53)45(8)25-33-42-20-22-55-33/h12-14,17-18,20,22,26-28,30-32,35-36H,11,15-16,19,21,23-25H2,1-10H3,(H,43,51)(H,44,50)/t30-,31-,32-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Chlorocebus aethiops) | BDBM50450892

(CHEMBL4207917)Show SMILES CCCC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)Cc1nccs1 |r| Show InChI InChI=1S/C41H63N7O6S/c1-11-16-33(49)44-35(27(4)5)40(53)48-21-15-19-31(48)39(52)46(9)32(24-29-17-13-12-14-18-29)37(50)43-30(23-26(2)3)38(51)47(10)36(28(6)7)41(54)45(8)25-34-42-20-22-55-34/h12-14,17-18,20,22,26-28,30-32,35-36H,11,15-16,19,21,23-25H2,1-10H3,(H,43,50)(H,44,49)/t30-,31-,32-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as reduction in neutral lipid synthesis incubated for 6 hrs using [1-14C]ole... |
J Nat Prod 80: 1161-1166 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00137
BindingDB Entry DOI: 10.7270/Q2251MS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data