

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035500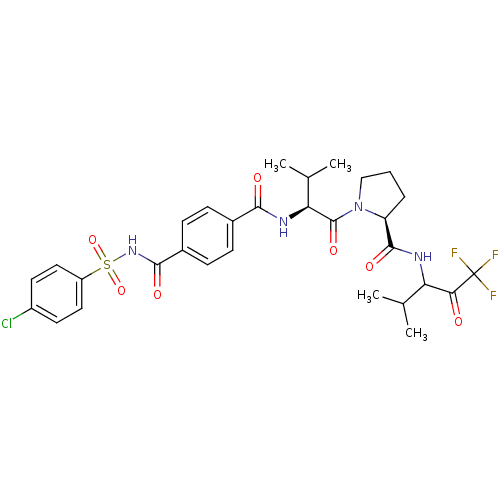 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035528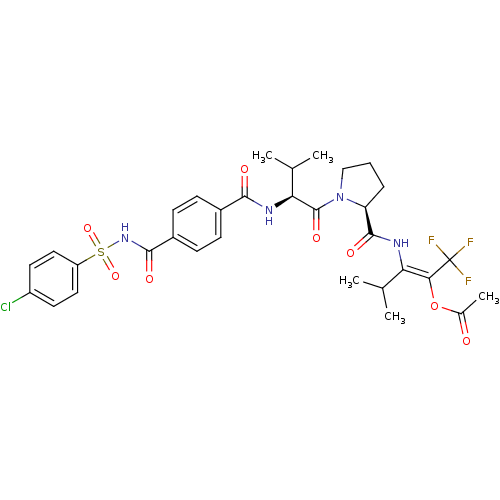 (Acetic acid (E)-2-[((S)-1-{(S)-2-[4-(4-chloro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035498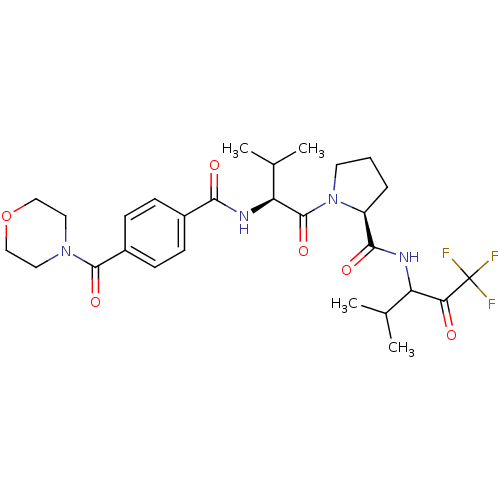 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035535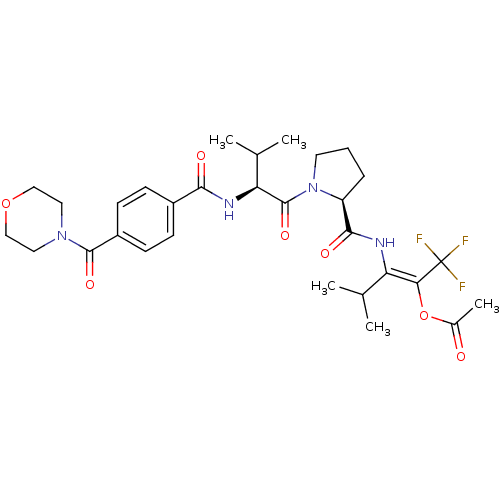 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065147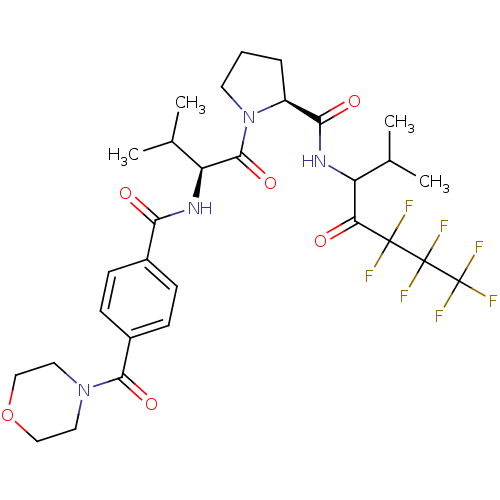 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495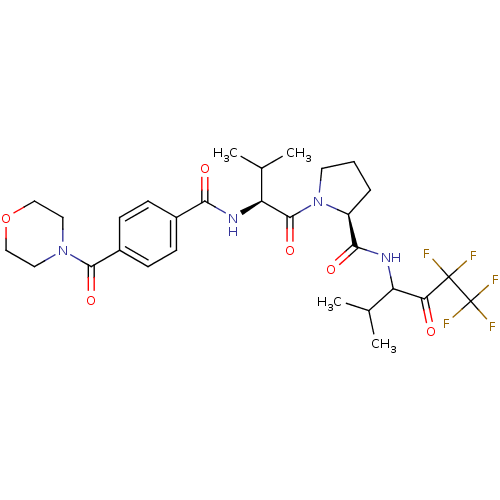 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065158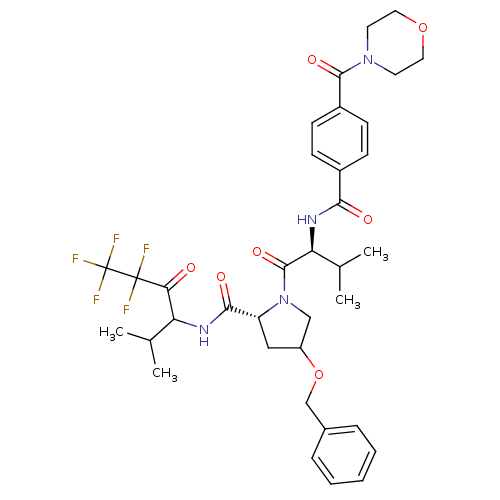 ((R)-4-Benzyloxy-1-{(S)-3-methyl-2-[4-(morpholine-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035531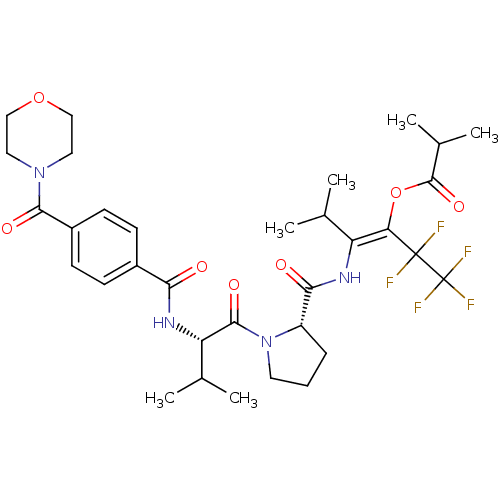 (CHEMBL74332 | Isobutyric acid (E)-3-methyl-2-[((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035530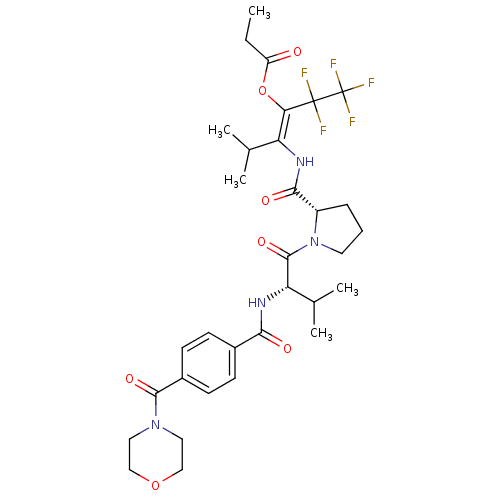 (CHEMBL74651 | Propionic acid (E)-3-methyl-2-[((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035532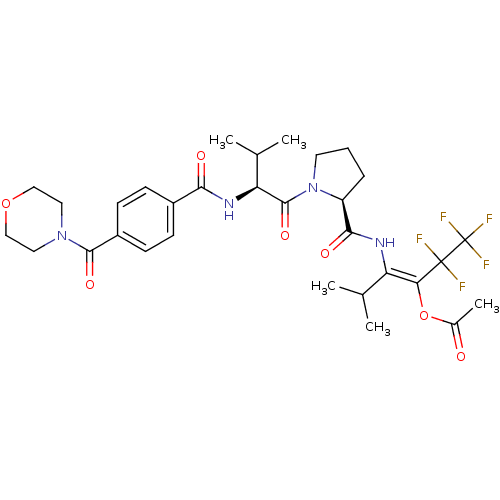 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065157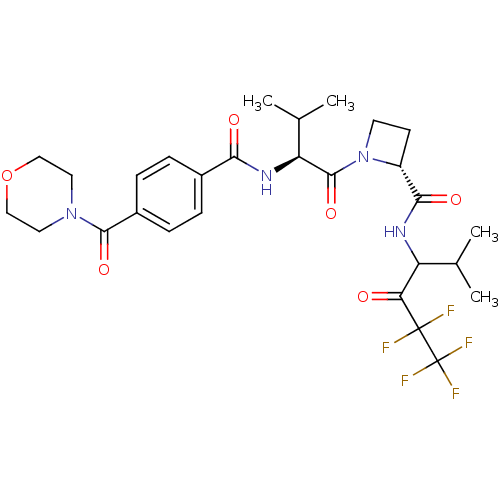 ((R)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065161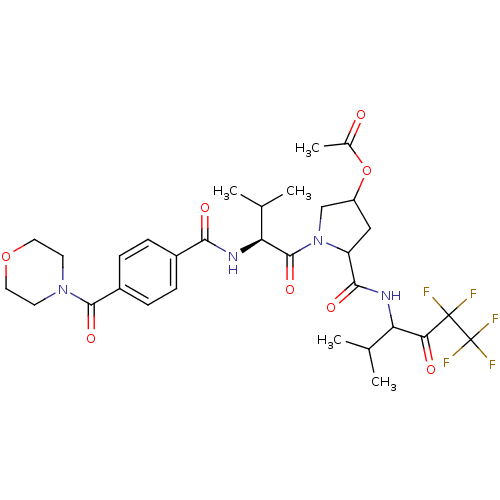 (Acetic acid 1-{(S)-3-methyl-2-[4-(morpholine-4-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065163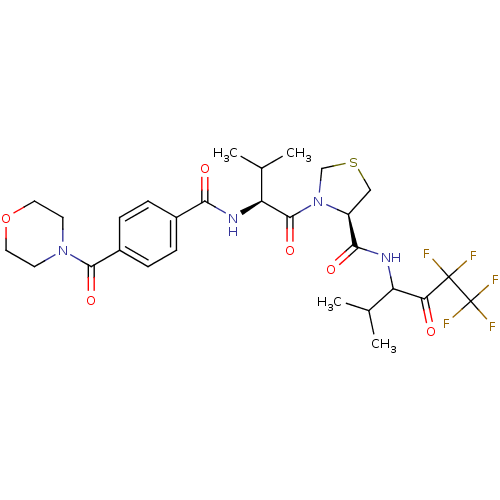 ((R)-3-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004711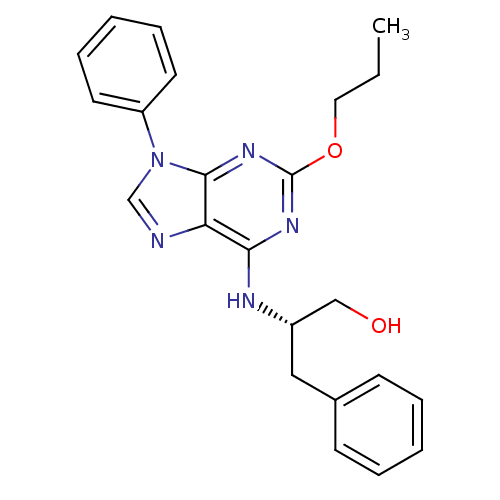 (3-Phenyl-2-(9-phenyl-2-propoxy-9H-purin-6-ylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065155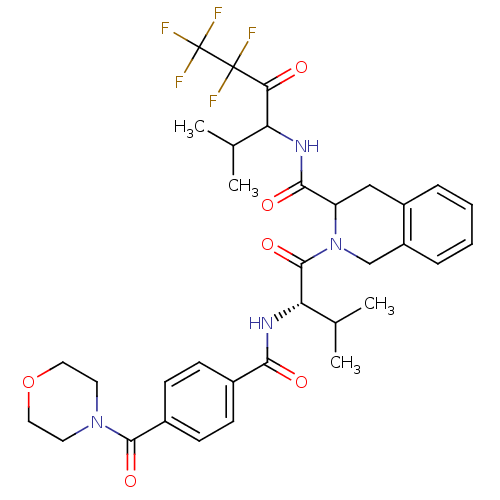 (2-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065154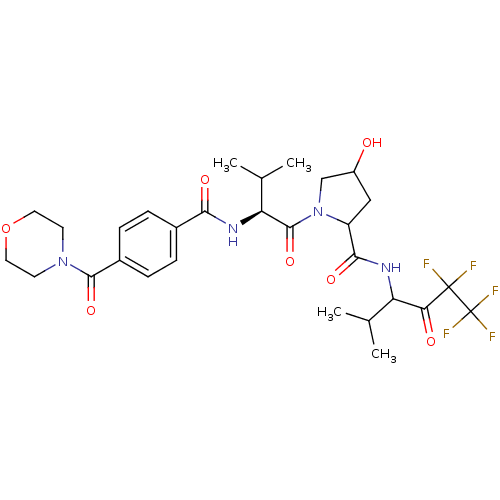 (4-Hydroxy-1-{(S)-3-methyl-2-[4-(morpholine-4-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065146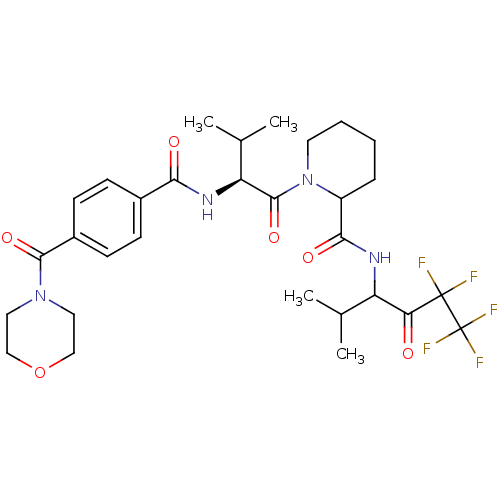 (1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035491 (CHEMBL59091 | Morpholine-4-carboxylic acid {(S)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065164 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50455626 (CHEMBL2112507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035528 (Acetic acid (E)-2-[((S)-1-{(S)-2-[4-(4-chloro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065156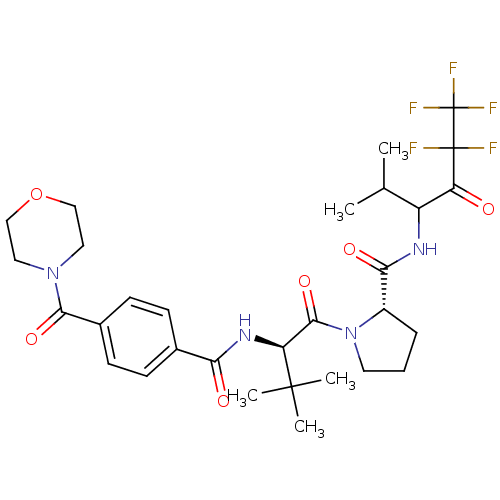 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50455625 (CHEMBL2111718) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50455625 (CHEMBL2111718) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50455625 (CHEMBL2111718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50455625 (CHEMBL2111718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004715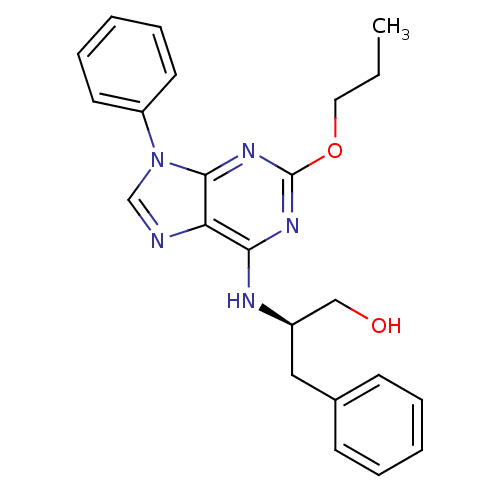 (3-Phenyl-2-(9-phenyl-2-propoxy-9H-purin-6-ylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004713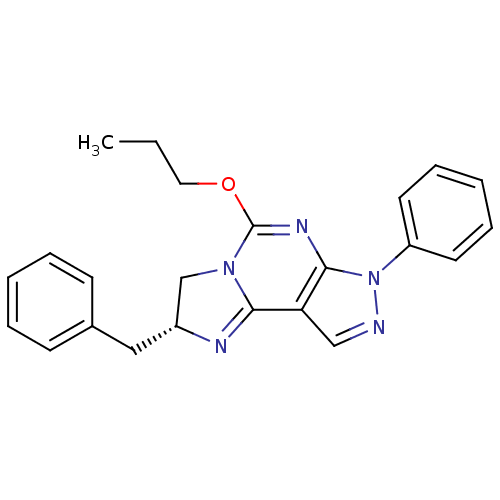 ((R)-2-Benzyl-7-phenyl-5-propoxy-2,7-dihydro-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50455626 (CHEMBL2112507) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065162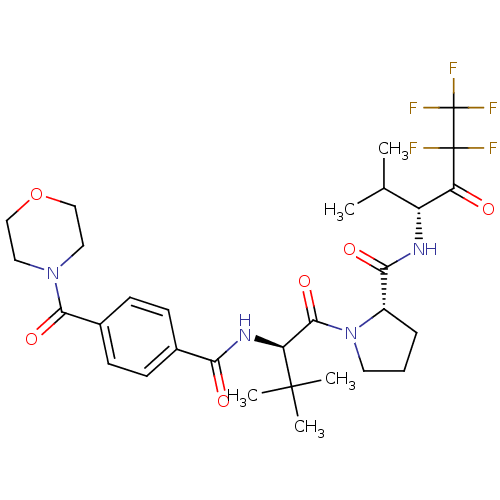 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50455624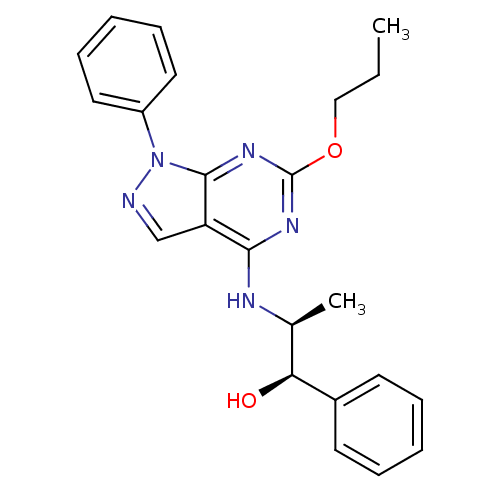 (CHEMBL2112500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004721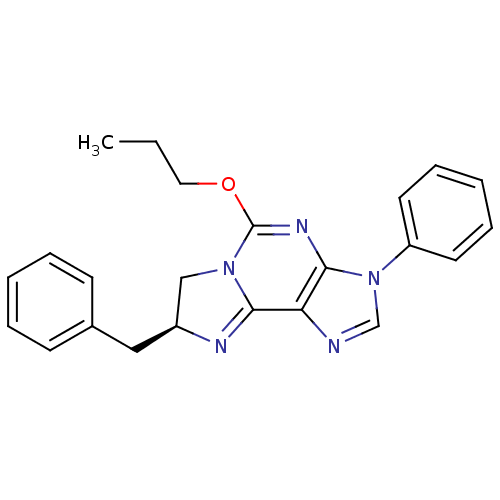 ((S)-8-Benzyl-3-phenyl-5-propoxy-7,8-dihydro-3H-imi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035532 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004722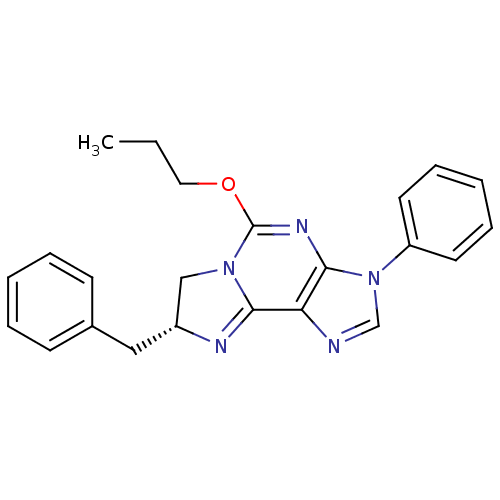 ((R)-8-Benzyl-3-phenyl-5-propoxy-7,8-dihydro-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004714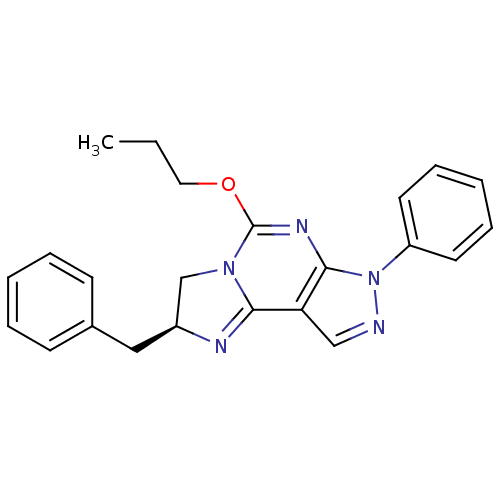 ((S)-2-Benzyl-7-phenyl-5-propoxy-2,7-dihydro-3H-imi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065153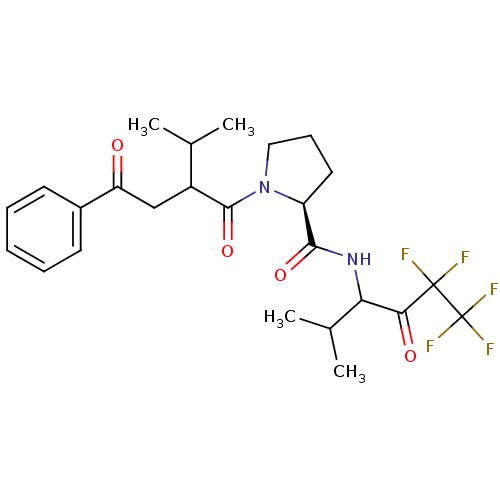 ((S)-1-[3-Methyl-2-(2-oxo-2-phenyl-ethyl)-butyryl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065151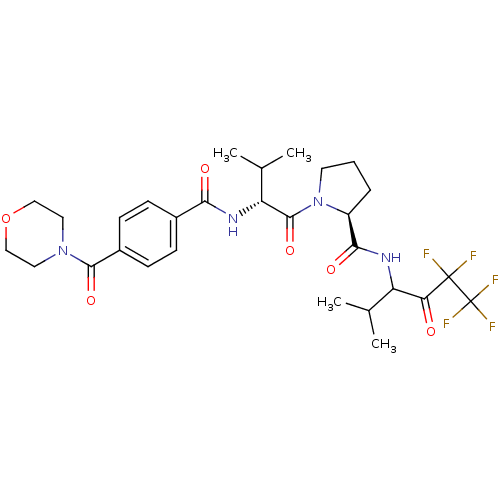 ((S)-1-{(R)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004711 (3-Phenyl-2-(9-phenyl-2-propoxy-9H-purin-6-ylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035533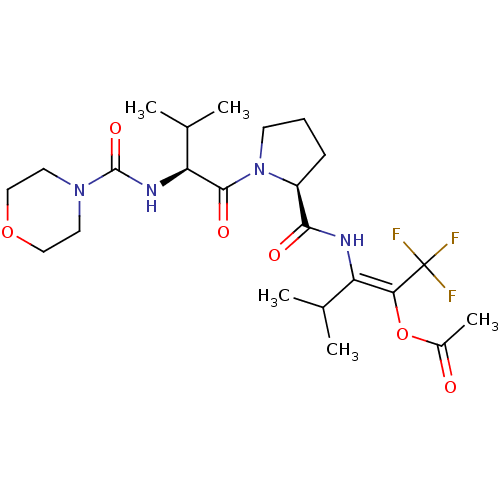 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035533 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50455624 (CHEMBL2112500) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004716 ((2R,3R)-2-Methyl-3,7-diphenyl-5-propoxy-2,7-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035536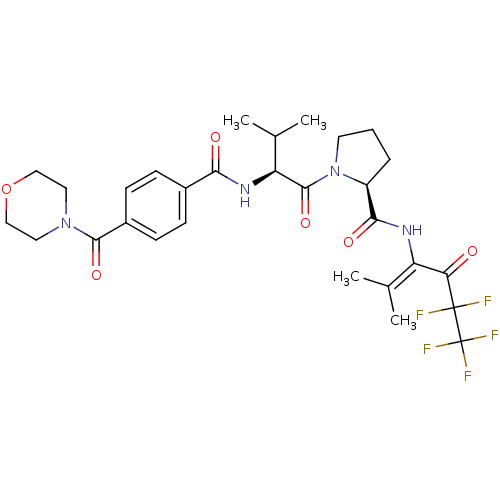 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004721 ((S)-8-Benzyl-3-phenyl-5-propoxy-7,8-dihydro-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50004714 ((S)-2-Benzyl-7-phenyl-5-propoxy-2,7-dihydro-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in whole rat brain membranes | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065150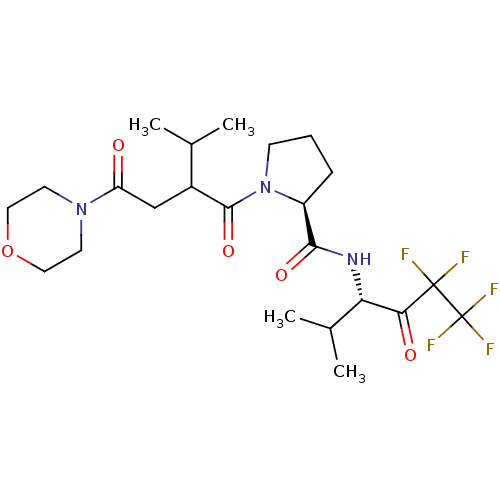 ((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004716 ((2R,3R)-2-Methyl-3,7-diphenyl-5-propoxy-2,7-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of of Bodipy-labelled cyclopamine binding to Smo expressed in COS-1 cells in presence of 20% NHS after 4 to 6 hrs by FACS flow cytometric ... | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065148 (4-(Morpholine-4-carbonyl)-N-{(S)-2-oxo-1-[(S)-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004715 (3-Phenyl-2-(9-phenyl-2-propoxy-9H-purin-6-ylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3263-9 (1992) BindingDB Entry DOI: 10.7270/Q2QF8RTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 741 total ) | Next | Last >> |