

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50148827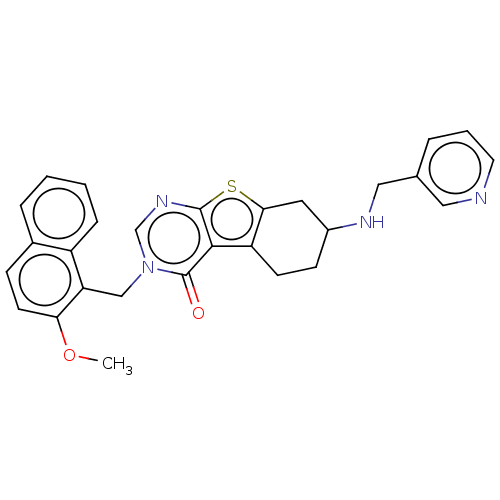 (CHEMBL3769975) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using tubulin-K40 peptide in prese... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant Pseudomonas aeruginosa MBL IMP-1 using nitrocefin as substrate preincubated for 20 mins by UV-spectrophotometric analysis | Bioorg Med Chem Lett 22: 6229-32 (2012) Article DOI: 10.1016/j.bmcl.2012.08.012 BindingDB Entry DOI: 10.7270/Q2CF9RCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50421220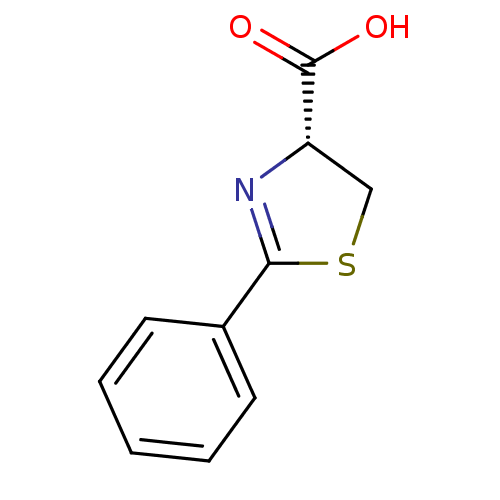 (CHEMBL2087628) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant Pseudomonas aeruginosa MBL IMP-1 using nitrocefin as substrate preincubated for 20 mins by UV-spectrophotometric analysis | Bioorg Med Chem Lett 22: 6229-32 (2012) Article DOI: 10.1016/j.bmcl.2012.08.012 BindingDB Entry DOI: 10.7270/Q2CF9RCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus anthracis) | BDBM50421214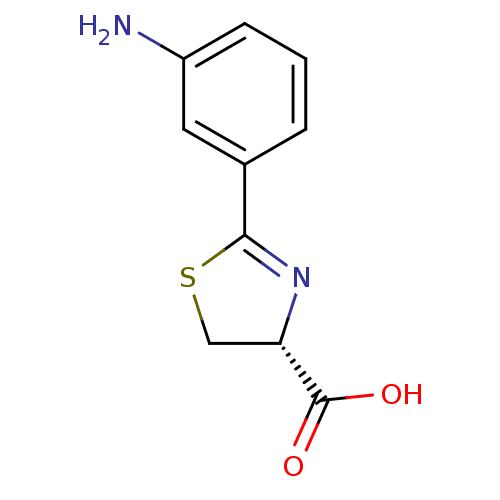 (CHEMBL2087633) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis MBL Bla2 using nitrocefin as substrate preincubated for 20 mins by UV-spectrophotometric analysis | Bioorg Med Chem Lett 22: 6229-32 (2012) Article DOI: 10.1016/j.bmcl.2012.08.012 BindingDB Entry DOI: 10.7270/Q2CF9RCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus anthracis) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis MBL Bla2 using nitrocefin as substrate preincubated for 20 mins by UV-spectrophotometric analysis | Bioorg Med Chem Lett 22: 6229-32 (2012) Article DOI: 10.1016/j.bmcl.2012.08.012 BindingDB Entry DOI: 10.7270/Q2CF9RCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM84558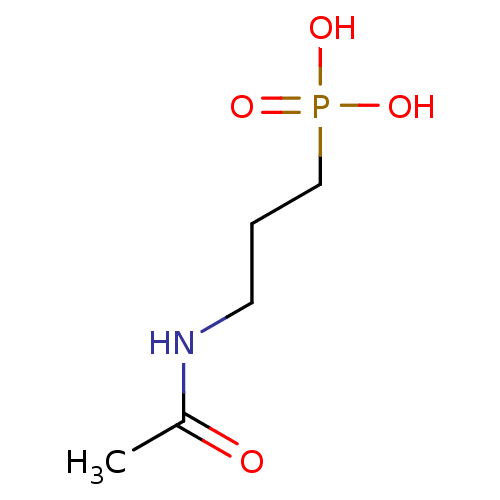 (Phosphate analogue, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 6.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50353128 (CHEMBL1231795) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using [histone H3 1 to 25 residues] and SAM substrate by scintillation proximity assay | Medchemcomm 5: 1821-1828 (2014) Article DOI: 10.1039/c4md00274a BindingDB Entry DOI: 10.7270/Q2V127VK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50028309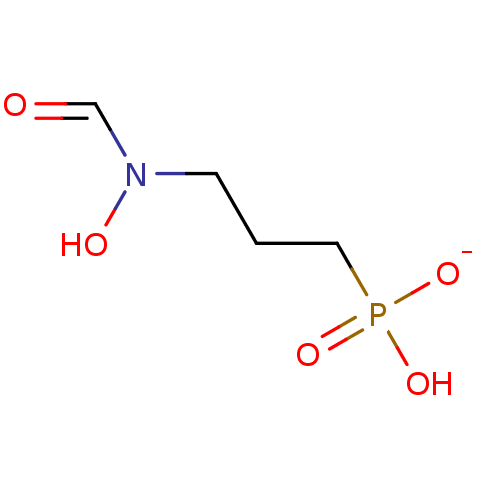 (Fosmidomycin Sodium) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50498658 (CHEMBL3621579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using [histone H3 1 to 25 residues] and SAM substrate by scintillation proximity assay | Medchemcomm 5: 1821-1828 (2014) Article DOI: 10.1039/c4md00274a BindingDB Entry DOI: 10.7270/Q2V127VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50181153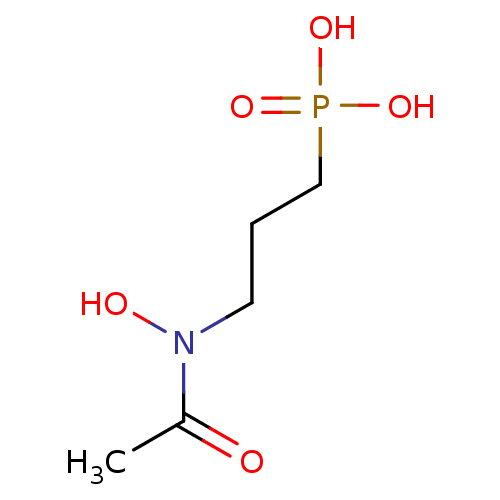 (3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50498656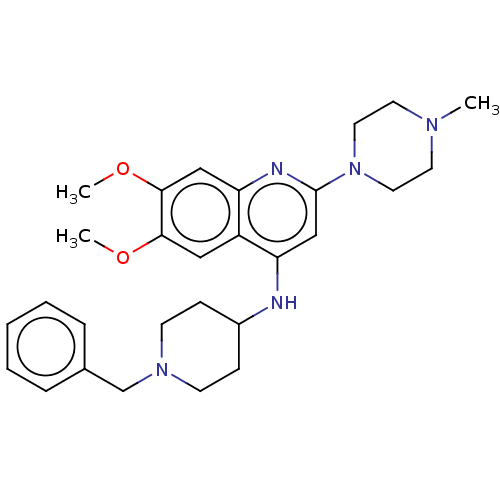 (CHEMBL3621580) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using [histone H3 1 to 25 residues] and SAM substrate by scintillation proximity assay | Medchemcomm 5: 1821-1828 (2014) Article DOI: 10.1039/c4md00274a BindingDB Entry DOI: 10.7270/Q2V127VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50380000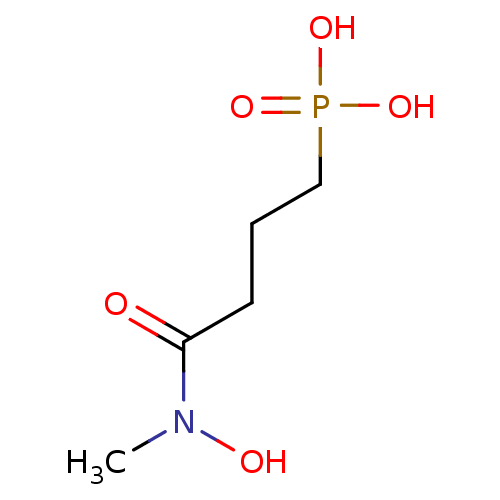 (CHEMBL258981) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonism of norepinephrine induced contraction of rat isolated thoracic aorta. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50028309 (Fosmidomycin Sodium) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonism of norepinephrine induced contraction of rat isolated thoracic aorta. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300028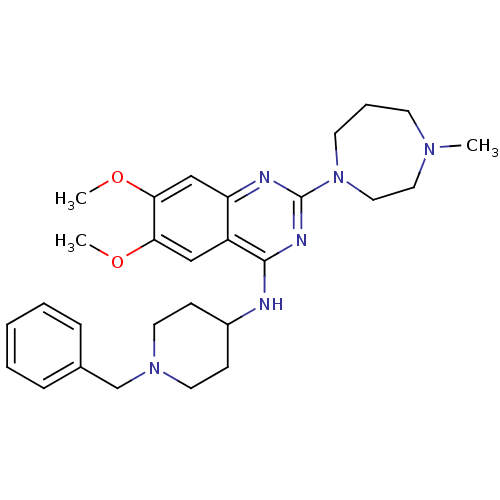 (CHEMBL569864 | N-(1-benzylpiperidin-4-yl)-6,7-dime...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using [histone H3 1 to 25 residues] and SAM substrate by scintillation proximity assay | Medchemcomm 5: 1821-1828 (2014) Article DOI: 10.1039/c4md00274a BindingDB Entry DOI: 10.7270/Q2V127VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50028309 (Fosmidomycin Sodium) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50265301 (5-(4-phenoxybenzylidene)pyrimidine-2,4,6(1H,3H,5H)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay | Bioorg Med Chem Lett 18: 4959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.028 BindingDB Entry DOI: 10.7270/Q2X066VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50498655 (CHEMBL1895209) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using [histone H3 1 to 25 residues] and SAM substrate by scintillation proximity assay | Medchemcomm 5: 1821-1828 (2014) Article DOI: 10.1039/c4md00274a BindingDB Entry DOI: 10.7270/Q2V127VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50181153 (3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonism of phenylephrine stimulated contraction of the rat isolated spleen. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50148827 (CHEMBL3769975) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli in presence of NAD+ by enzyme coup... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50379999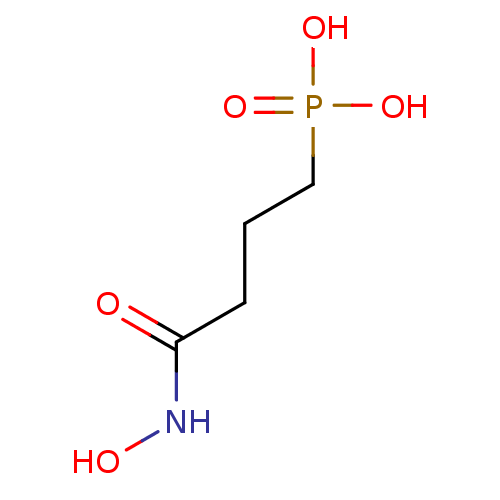 (CHEMBL1161784) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonism of norepinephrine induced contraction of rat isolated thoracic aorta. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50265171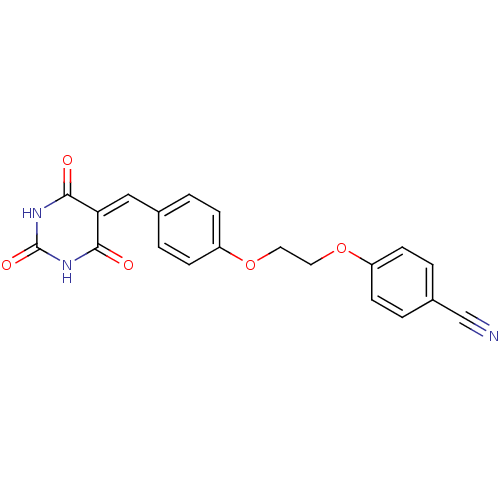 (4-(4-((2,4,6-trioxotetrahydropyrimidin-5(6H)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay | Bioorg Med Chem Lett 18: 4959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.028 BindingDB Entry DOI: 10.7270/Q2X066VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Rattus norvegicus) | BDBM50271605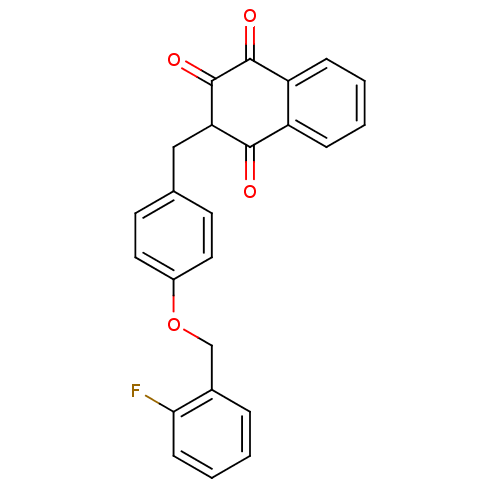 (2-(4-(2-fluorobenzyloxy)benzyl)-3-hydroxynaphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosigliatzone from PPARgamma in rat adipocytes | Bioorg Med Chem Lett 18: 3192-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.072 BindingDB Entry DOI: 10.7270/Q2J9665P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50265213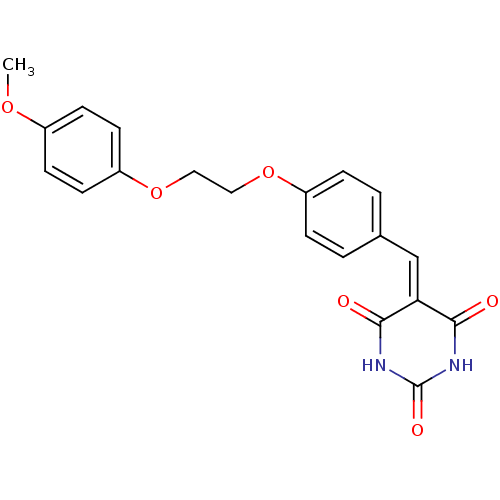 (5-(4-((4-methoxyphenoxy)methyl)benzylidene)pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay | Bioorg Med Chem Lett 18: 4959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.028 BindingDB Entry DOI: 10.7270/Q2X066VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501437 (CHEMBL4101347) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50148781 (CHEMBL3770903) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of human SIRT2 (25 to 389 residues) using (Z-Lys(Acetyl)-AMC) as substrate after 4 hrs in presence of NAD+ by fluorescence assay | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50498659 (CHEMBL1902352) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using [histone H3 1 to 25 residues] and SAM substrate by scintillation proximity assay | Medchemcomm 5: 1821-1828 (2014) Article DOI: 10.1039/c4md00274a BindingDB Entry DOI: 10.7270/Q2V127VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50265270 (5-(4-((10H-phenoxazin-10-yl)methyl)benzylidene)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay | Bioorg Med Chem Lett 18: 4959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.028 BindingDB Entry DOI: 10.7270/Q2X066VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501456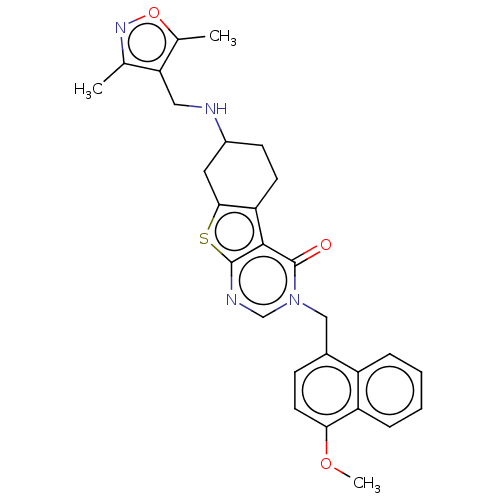 (CHEMBL4088755) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase Su(var)3-9 (Drosophila melanogaster) | BDBM50315537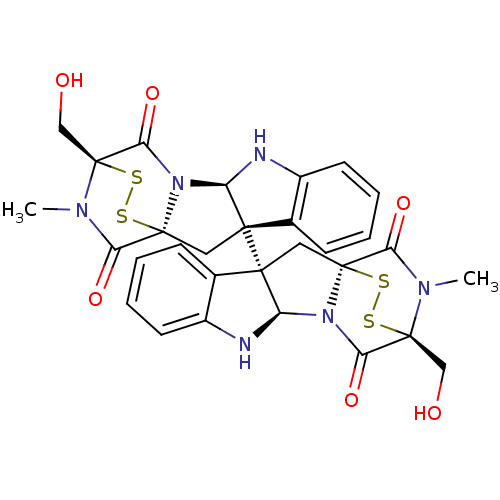 (CHEMBL1089316 | chaetocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of Drosophila melanogaster Histone-lysine N-methyltransferase Su(var)3-9 | J Med Chem 56: 8616-25 (2013) Article DOI: 10.1021/jm401063r BindingDB Entry DOI: 10.7270/Q2NS0WDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501455 (CHEMBL4091659) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Rattus norvegicus) | BDBM50049240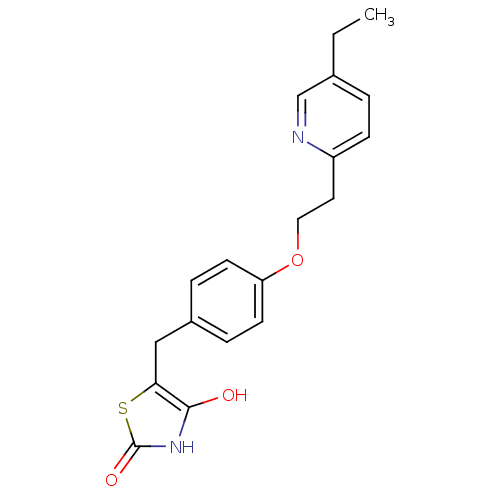 ((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosigliatzone from PPARgamma in rat adipocytes | Bioorg Med Chem Lett 18: 3192-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.072 BindingDB Entry DOI: 10.7270/Q2J9665P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50049240 ((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay | Bioorg Med Chem Lett 18: 4959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.028 BindingDB Entry DOI: 10.7270/Q2X066VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501451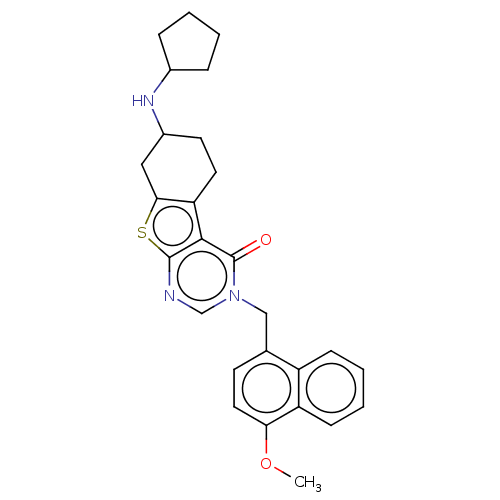 (CHEMBL4078889) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H1 (Homo sapiens (Human)) | BDBM50315537 (CHEMBL1089316 | chaetocin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human Histone-lysine N-methyltransferase SUV39H1 | J Med Chem 56: 8616-25 (2013) Article DOI: 10.1021/jm401063r BindingDB Entry DOI: 10.7270/Q2NS0WDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50265211 (5-(3-(2-(p-tolyloxy)ethoxy)benzylidene)pyrimidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay | Bioorg Med Chem Lett 18: 4959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.028 BindingDB Entry DOI: 10.7270/Q2X066VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501499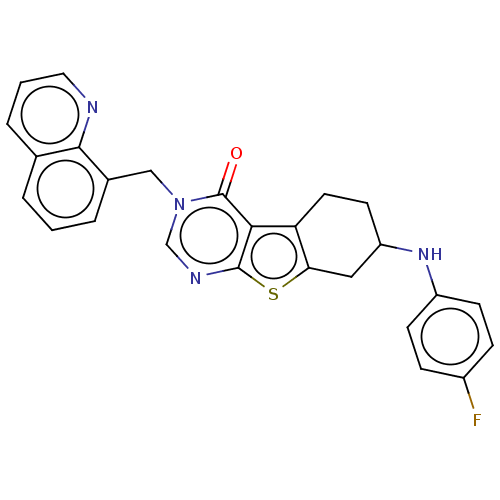 (CHEMBL4064852) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501453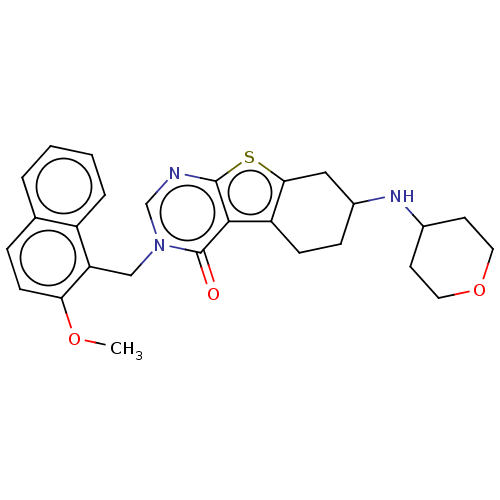 (CHEMBL4094485) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501450 (CHEMBL4091606) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501494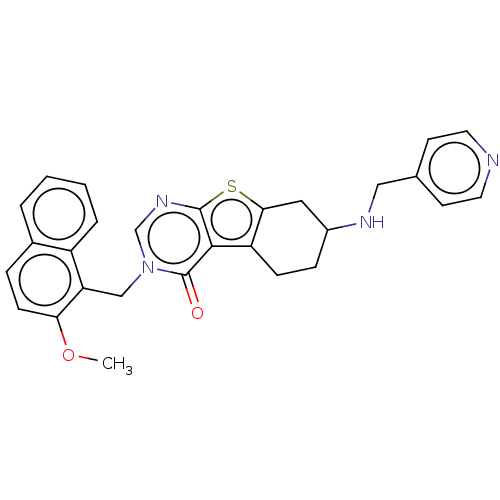 (CHEMBL4105292) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501435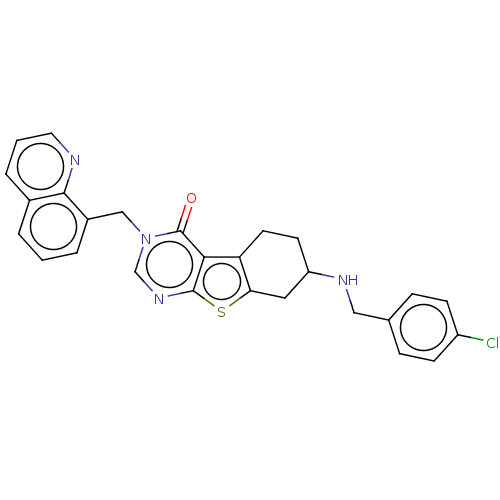 (CHEMBL4078845) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50148827 (CHEMBL3769975) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501452 (CHEMBL4073924) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501485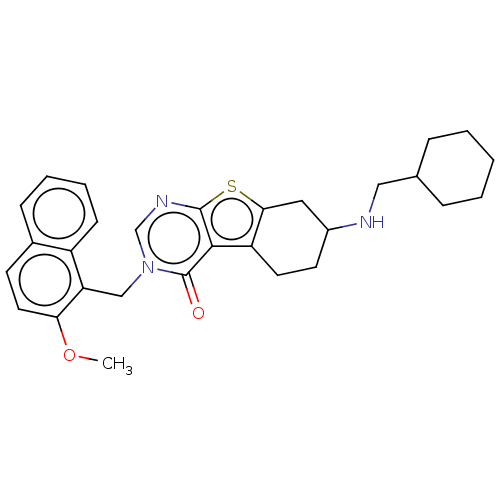 (CHEMBL4061689) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501507 (CHEMBL4093162) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501472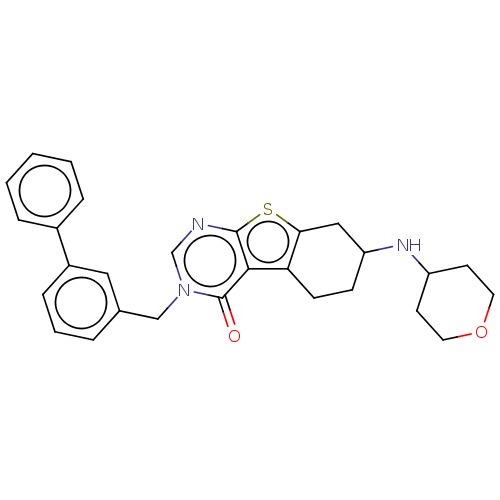 (CHEMBL4092831) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501491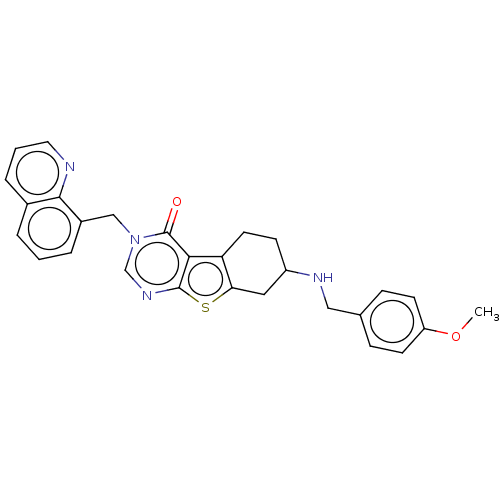 (CHEMBL4094149) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501510 (CHEMBL4071269) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501481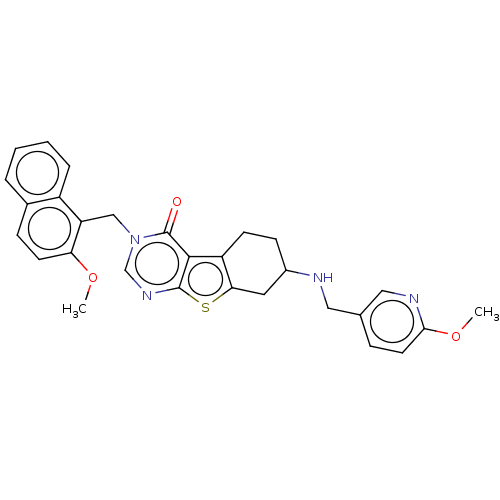 (CHEMBL4104283) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Rattus norvegicus) | BDBM50271732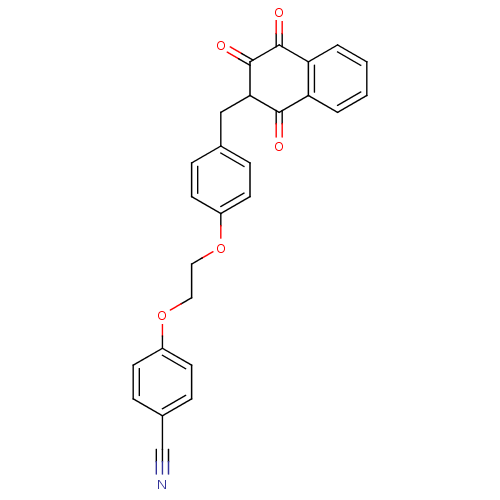 (4-(2-(4-((3-hydroxy-1,4-dioxo-1,4-dihydronaphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]rosigliatzone from PPARgamma in rat adipocytes | Bioorg Med Chem Lett 18: 3192-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.072 BindingDB Entry DOI: 10.7270/Q2J9665P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50501457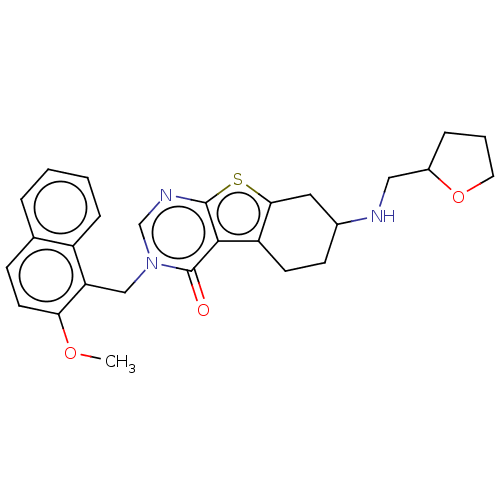 (CHEMBL4084437) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using p53 derived (379 to 382 resi... | J Med Chem 60: 1928-1945 (2017) Article DOI: 10.1021/acs.jmedchem.6b01690 BindingDB Entry DOI: 10.7270/Q23F4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 191 total ) | Next | Last >> |