

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM123407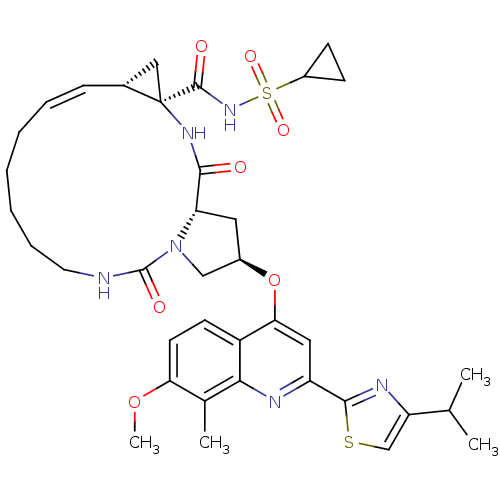 (US8741926, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410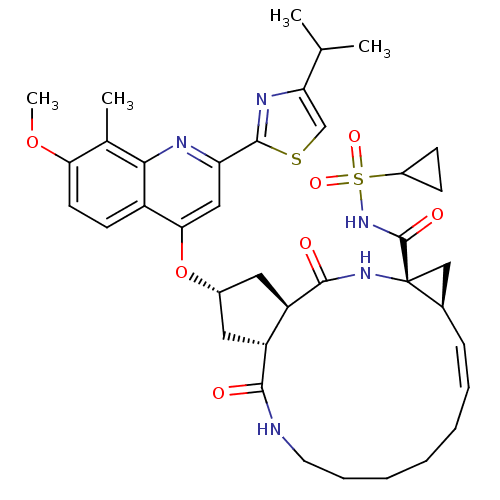 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413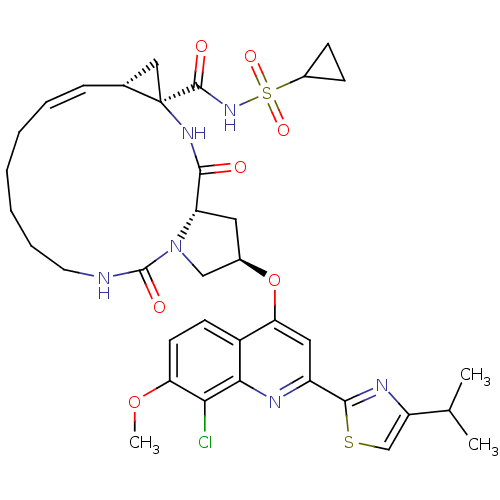 (US8741926, 94 | US8754106, 94) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123411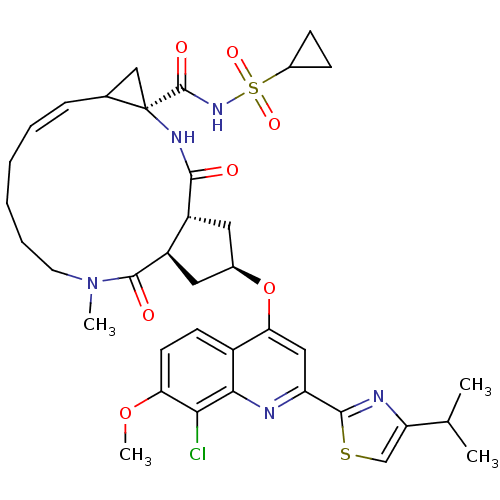 (US8741926, 56) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415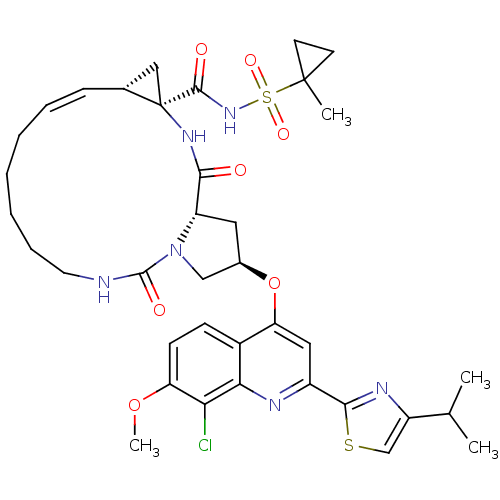 (US8741926, 95 | US8754106, 95) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415 (US8741926, 95 | US8754106, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413 (US8741926, 94 | US8754106, 94) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124106 (US8754106, 56) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414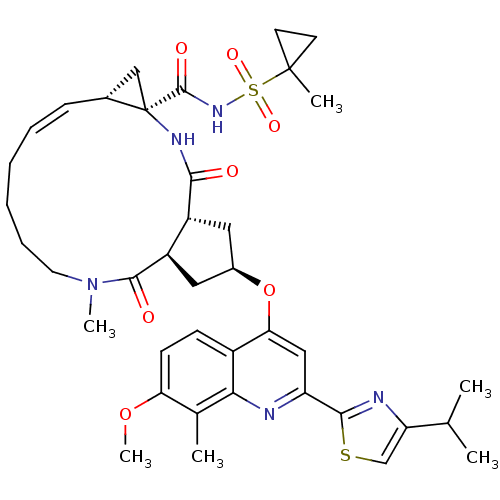 (US8741926, 48 | US8754106, 48) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414 (US8741926, 48 | US8754106, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412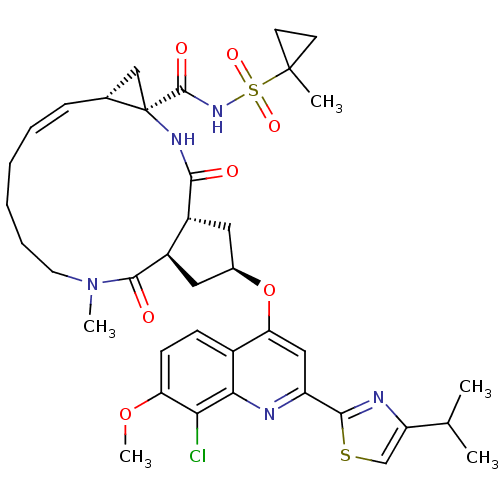 (US8741926, 57 | US8754106, 57) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412 (US8741926, 57 | US8754106, 57) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124105 (US8754106, 55) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123408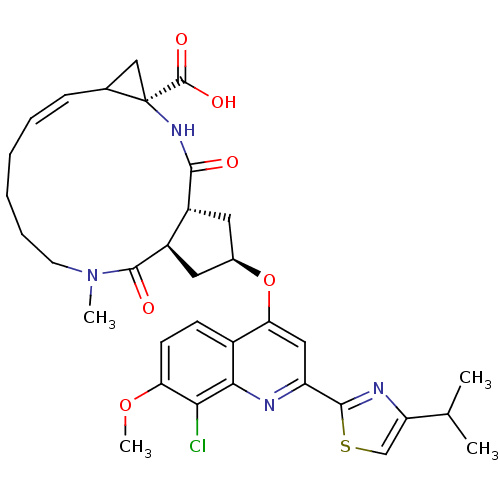 (US8741926, 55) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123409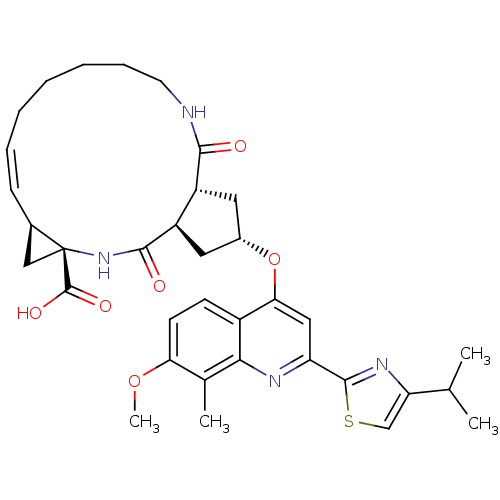 (US8741926, 81) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50410310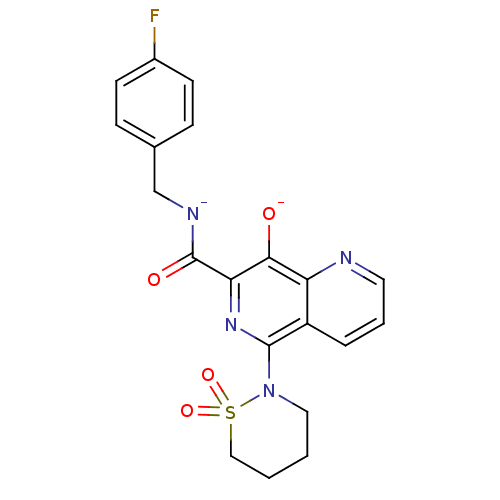 (CHEMBL2079773) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50410311 (CHEMBL2068731) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422732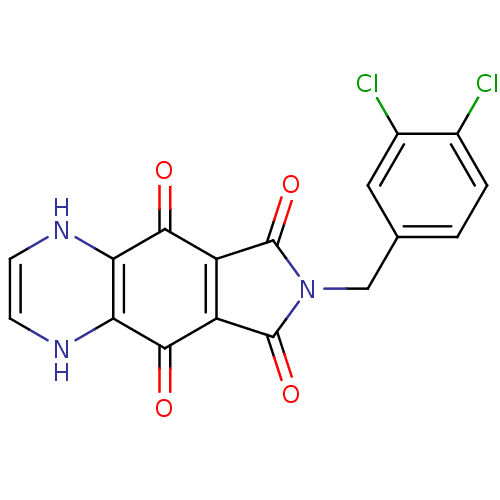 (CHEMBL424782) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422734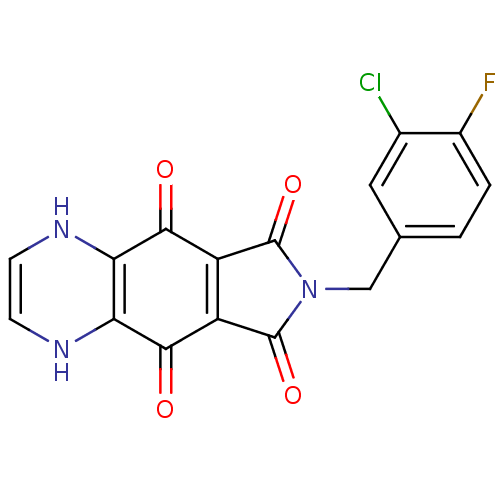 (CHEMBL368301) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422722 (CHEMBL360752) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422731 (CHEMBL425516) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422706 (CHEMBL367104) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422718 (CHEMBL177640) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422733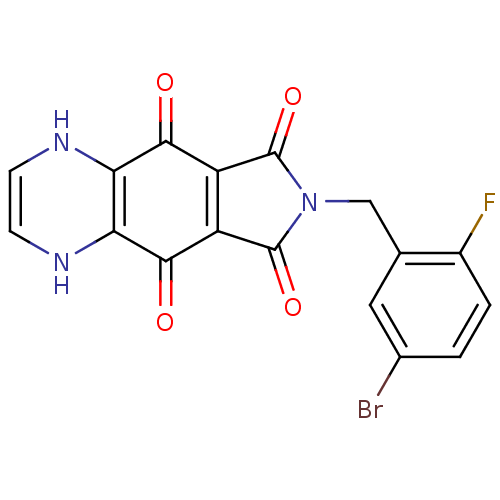 (CHEMBL414654) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422697 (CHEMBL362252) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422703 (CHEMBL366982) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422711 (CHEMBL360274) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422699 (CHEMBL369841) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422721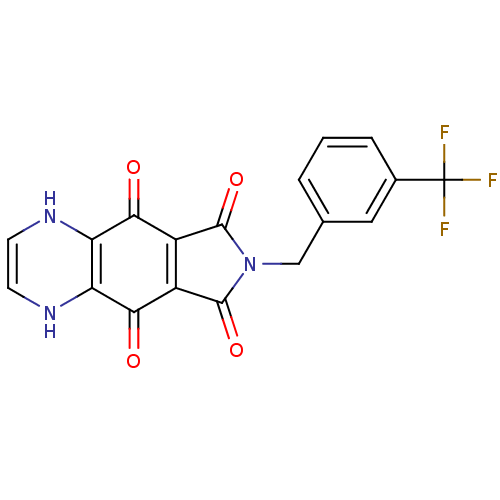 (CHEMBL177622) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422704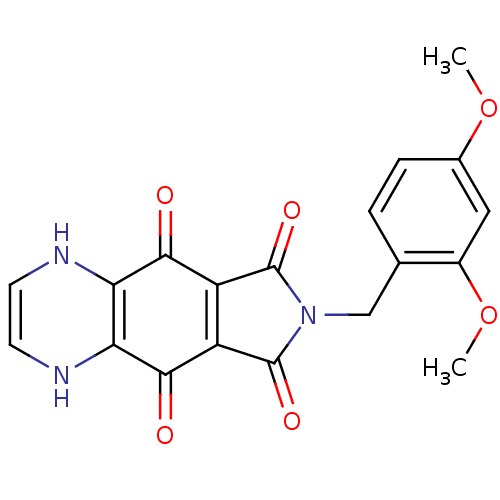 (CHEMBL177215) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422700 (CHEMBL177515) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422728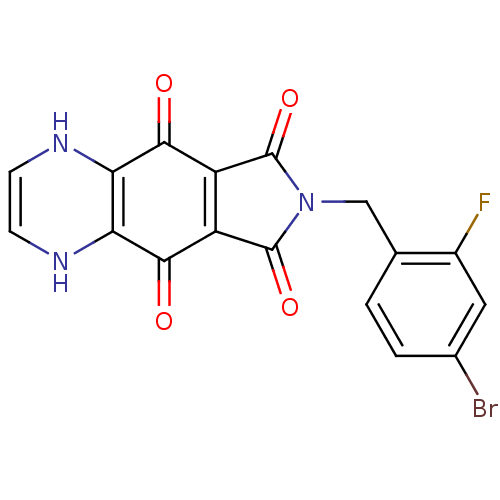 (CHEMBL424780) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422702 (CHEMBL174793) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422724 (CHEMBL177724) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422696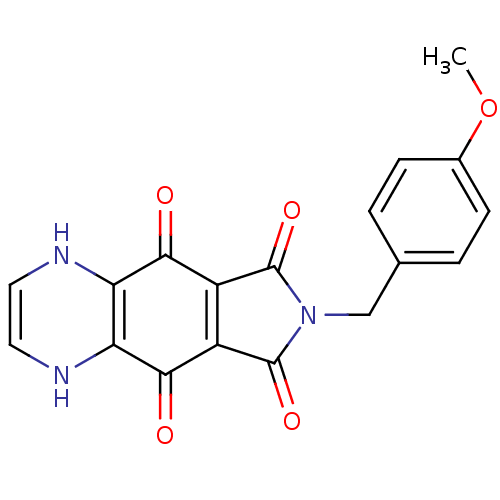 (CHEMBL174001) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422715 (CHEMBL177690) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422708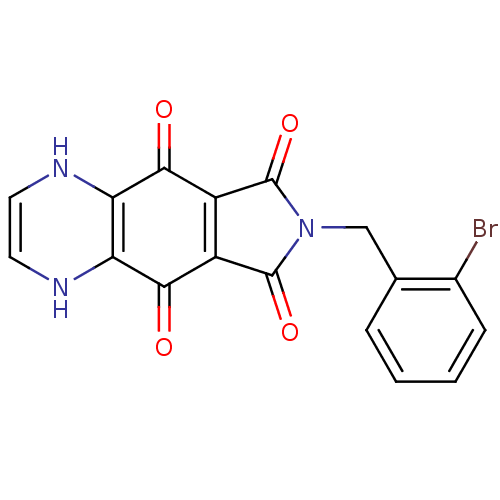 (CHEMBL177380) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422698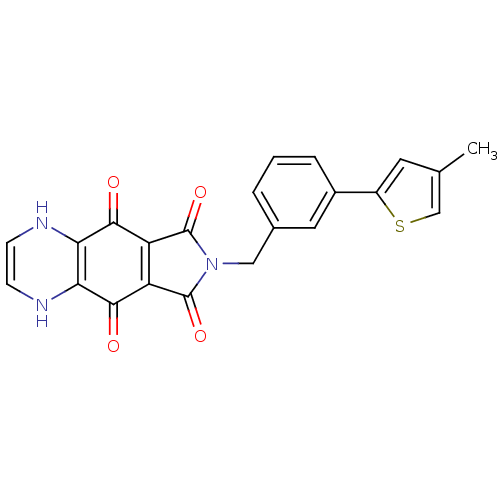 (CHEMBL368424) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422730 (CHEMBL177405) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 776 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422713 (CHEMBL177311) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 813 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422709 (CHEMBL174851) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422705 (CHEMBL368509) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422695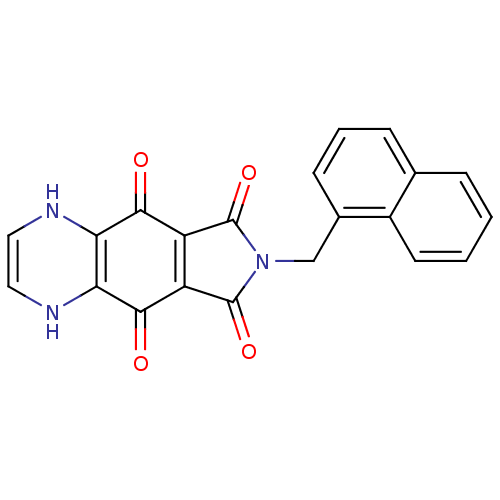 (CHEMBL175404) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422717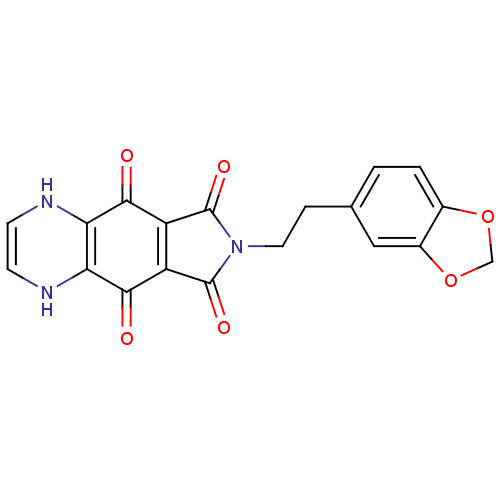 (CHEMBL175671) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422727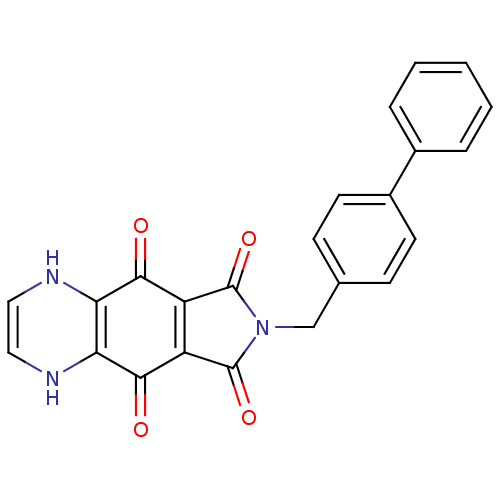 (CHEMBL367945) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50422712 (CHEMBL177704) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tibotec BVBA Curated by ChEMBL | Assay Description Log of inhibitory concentration against human immunodeficiency virus type 1 integrase | J Med Chem 48: 1930-40 (2005) Article DOI: 10.1021/jm049559q BindingDB Entry DOI: 10.7270/Q29Z94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |