

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9264 ((2R)-2-hydroxy-2-[(10S)-16-hydroxy-12-oxo-3,4,5,6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9262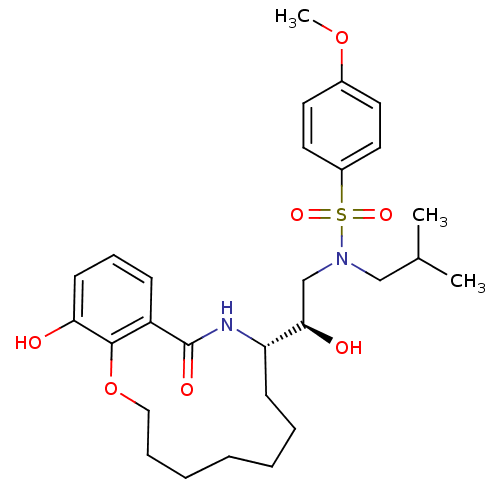 ((2R)-2-hydroxy-2-[(9S)-15-hydroxy-11-oxo-2,3,4,5,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50362863 (CHEMBL1940418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9260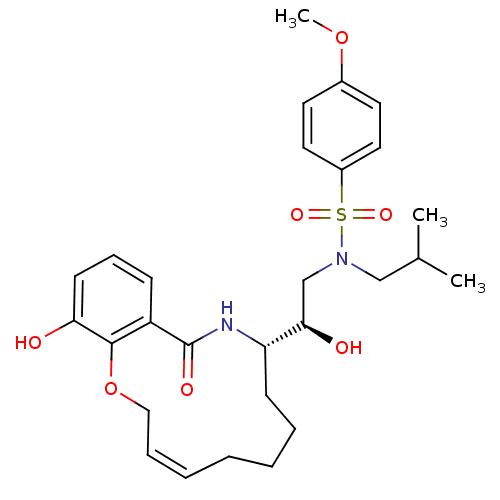 ((2R)-2-hydroxy-2-[(9S)-15-hydroxy-11-oxo-2,5,6,7,8...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9266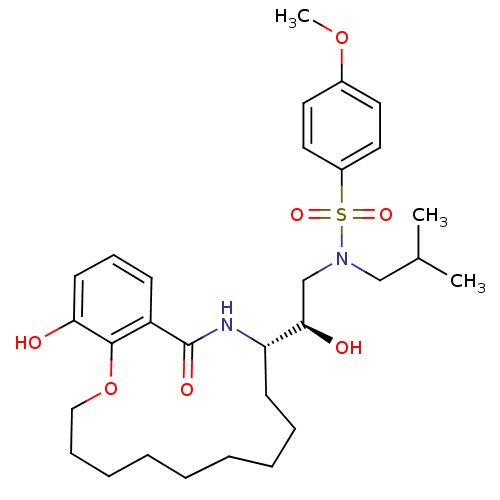 ((2R)-2-hydroxy-2-[(11S)-17-hydroxy-13-oxo-2,3,4,5,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9263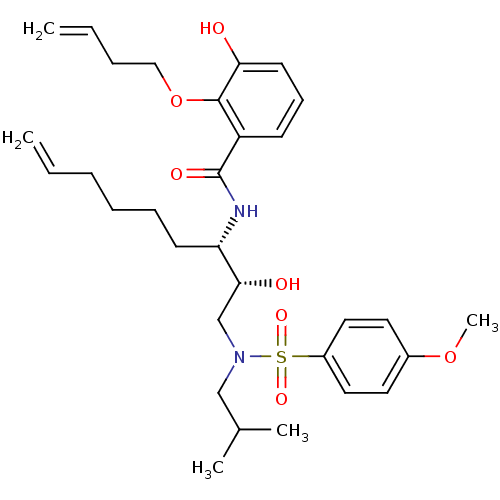 (2-(but-3-en-1-yloxy)-3-hydroxy-N-[(2R,3S)-2-hydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM563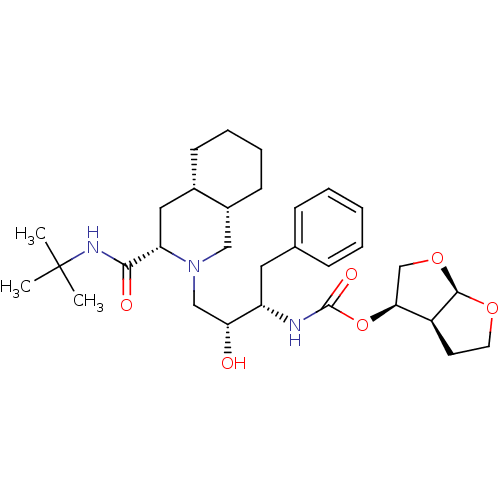 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl (1S,2R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568924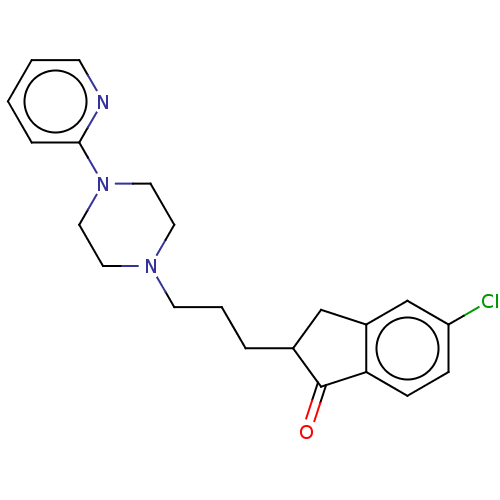 (CHEMBL4862180) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568924 (CHEMBL4862180) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568921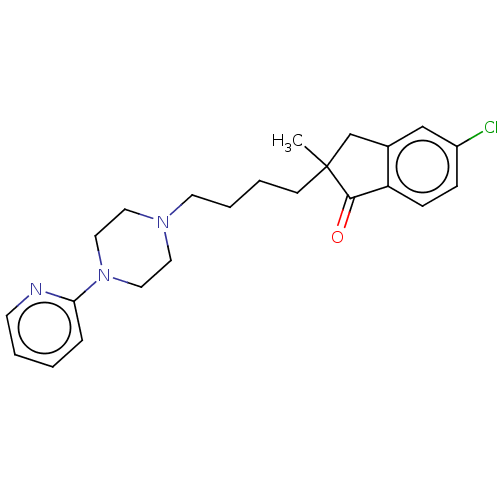 (CHEMBL4871868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568921 (CHEMBL4871868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9268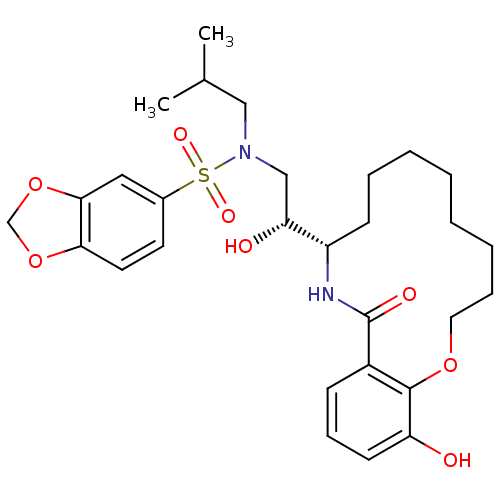 ((2R)-N-(2H-1,3-benzodioxol-5-yl)-2-hydroxy-2-[(10S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50362863 (CHEMBL1940418) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568919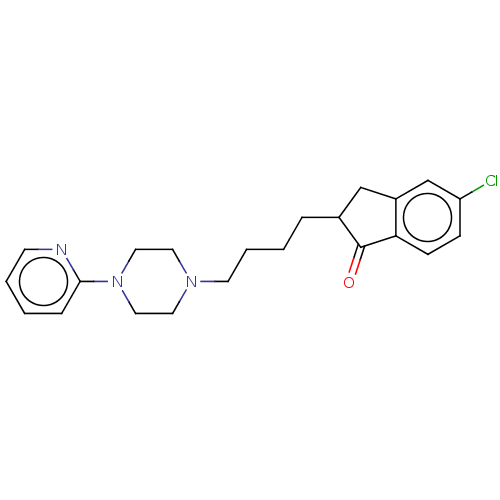 (CHEMBL4857665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568911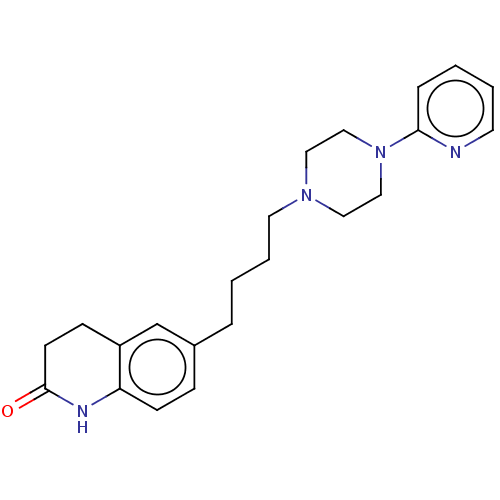 (CHEMBL4857597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568919 (CHEMBL4857665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568911 (CHEMBL4857597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568910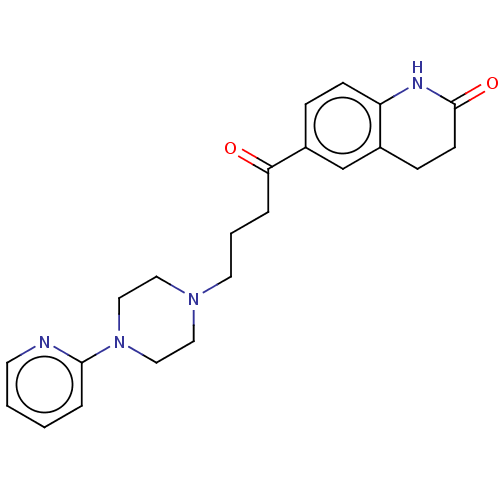 (CHEMBL4845947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568910 (CHEMBL4845947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568917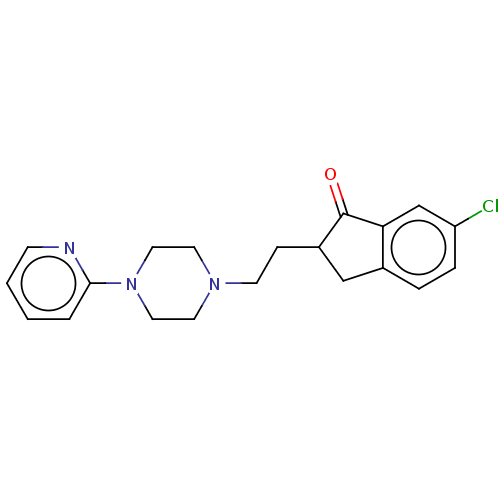 (CHEMBL4876297) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568917 (CHEMBL4876297) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568914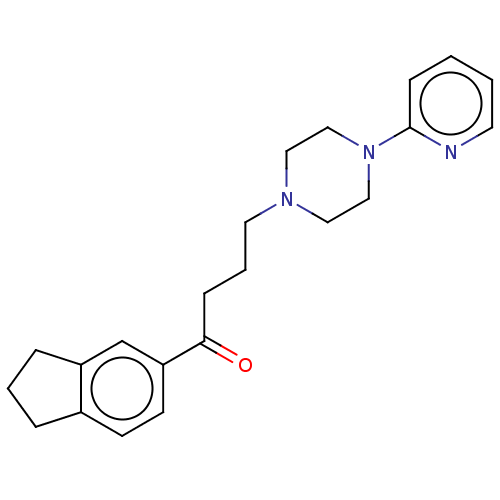 (CHEMBL4874069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568914 (CHEMBL4874069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9258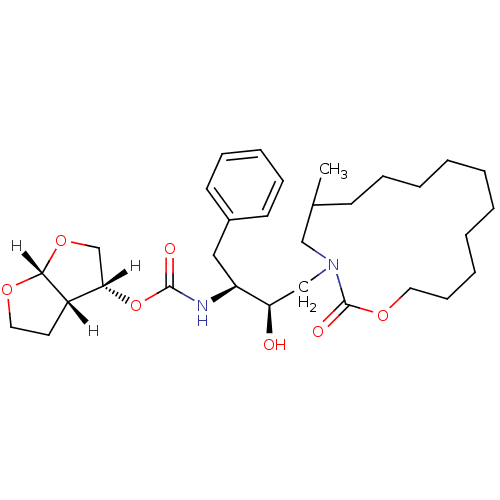 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | -46.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568916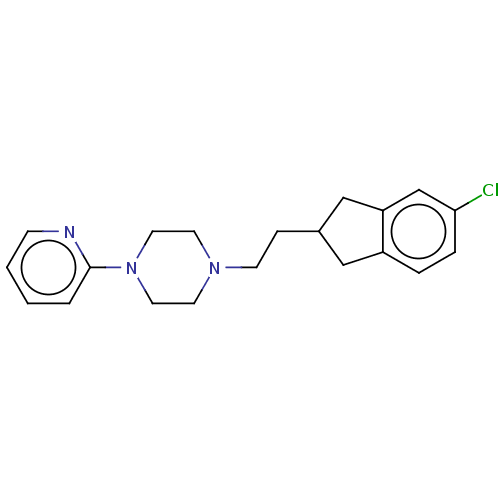 (CHEMBL4866058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568916 (CHEMBL4866058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50036733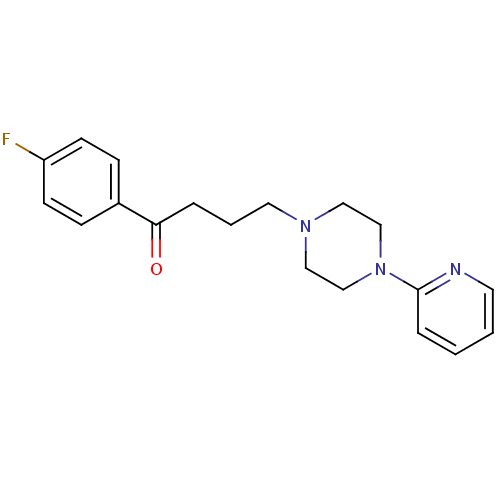 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50568915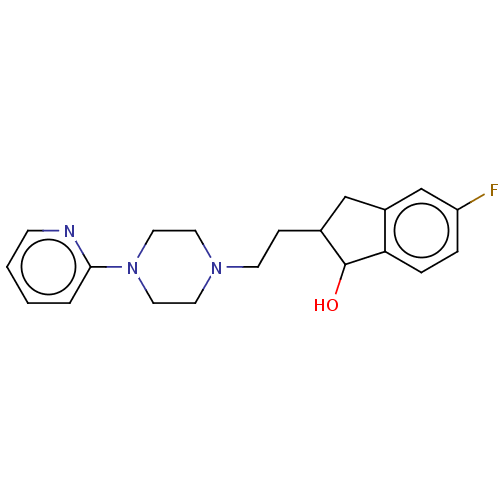 (CHEMBL4866053) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568915 (CHEMBL4866053) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ketanserin from human 5-HT2A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9265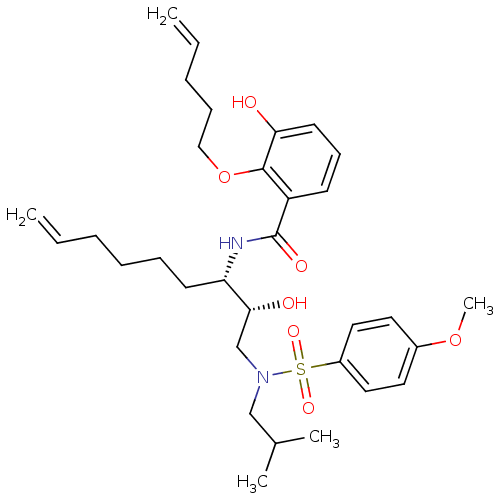 (3-hydroxy-N-[(1S)-1-((1R)-1-hydroxy-2-{isobutyl[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50568917 (CHEMBL4876297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50568917 (CHEMBL4876297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568923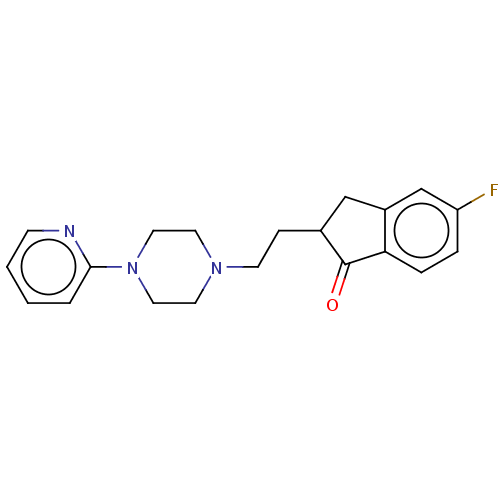 (CHEMBL4871850) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9257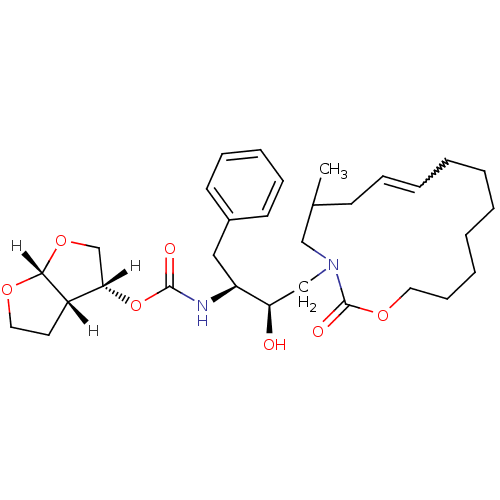 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9259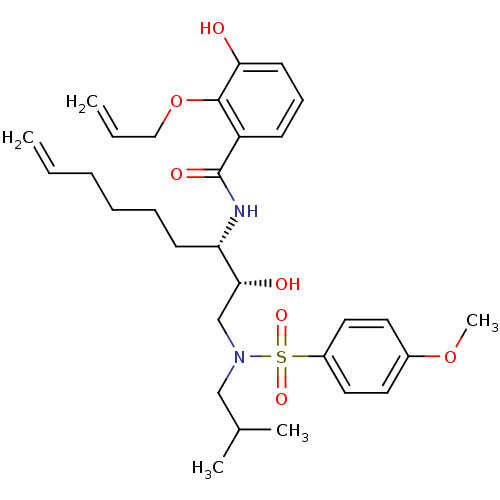 (2-(allyloxy)-3-hydroxy-N-[(1S)-1-((1R)-1-hydroxy-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | J Med Chem 48: 3576-85 (2005) Article DOI: 10.1021/jm050019i BindingDB Entry DOI: 10.7270/Q2NS0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568923 (CHEMBL4871850) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568909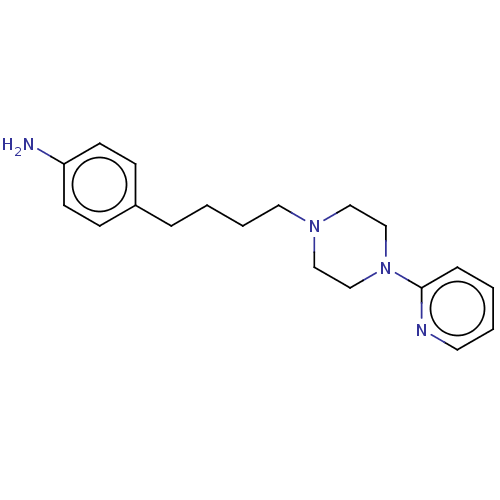 (CHEMBL4845741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 536 total ) | Next | Last >> |