

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM205460 (Salicyl-AMS (3)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM205461 (Salicyl-AMSN (4)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM205459 (Anthranilyl-AMSN (2)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM205458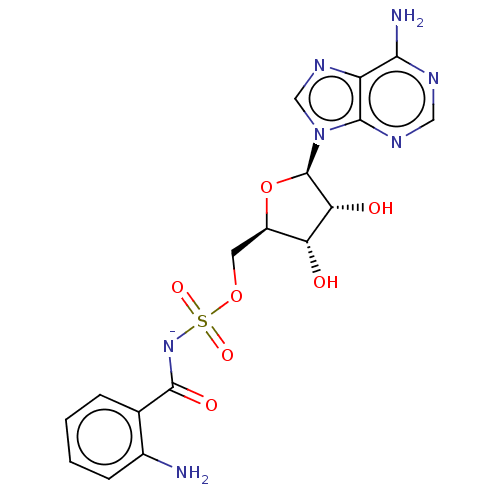 (Anthranilyl-AMS (1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50339905 (5'-O-(N-(L-CYSTEINYL)-SULFAMOYL)ADENOSINE | 5'-O-[...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM205462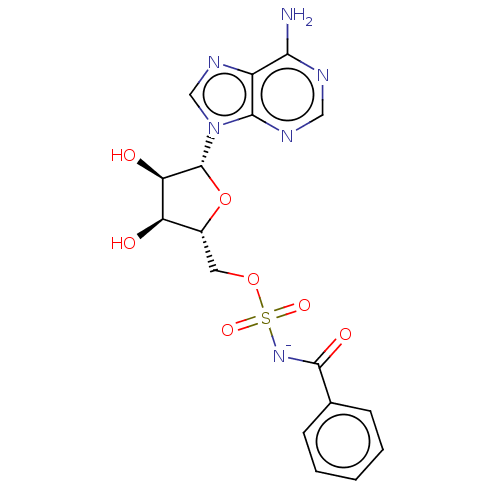 (Benzoyl-AMS (5)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197294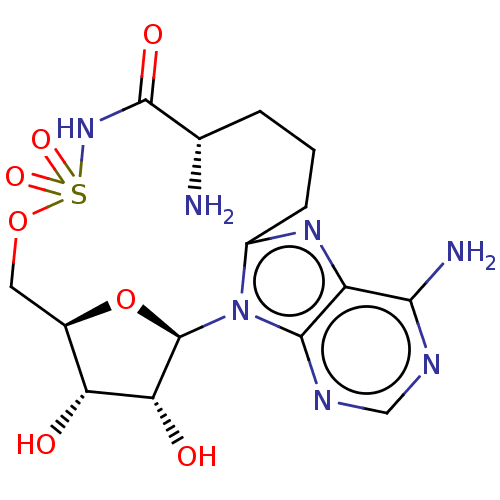 (CHEMBL3897138) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197291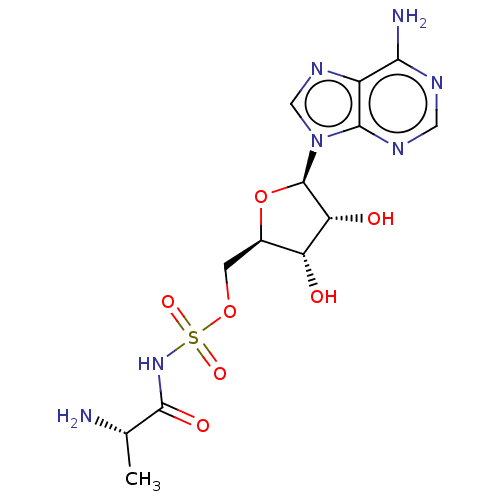 (AlaSA) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM50413189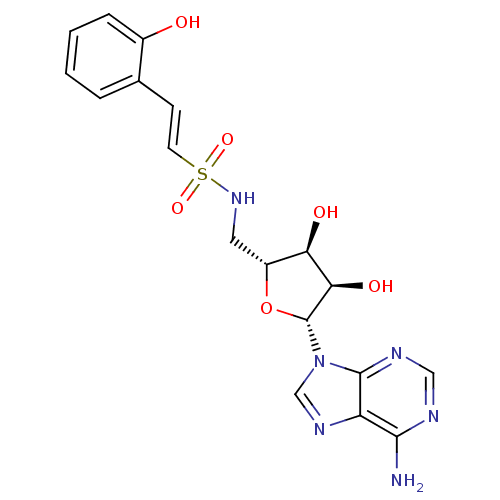 (CHEMBL457531 | Salicyl-AVSN (7)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate--CoA ligase (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM205463 (Anthranilyl-AVSN (6)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
East Carolina University | Assay Description Reactions were performed at 37 °C in a 0.5 mL volume in a Varian Cary 100 UV-visible spectrophotometer with Cary WinUV software. Reaction mixtures co... | ACS Chem Biol 11: 3061-3067 (2016) Article DOI: 10.1021/acschembio.6b00575 BindingDB Entry DOI: 10.7270/Q2M61J25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50324670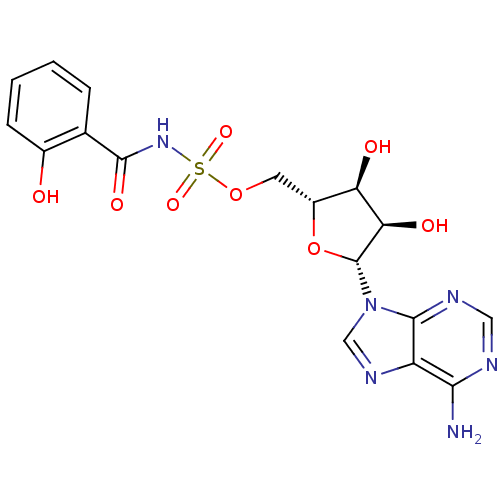 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Medical College of Cornell University Curated by ChEMBL | Assay Description Inhibition of adenylation activity of Mycobacterium tuberculosis MbtA after 30 mins by ATP-[32P]pyrophosphate exchange assay | Nat Chem Biol 1: 29-32 (2006) Article DOI: 10.1038/nchembio706 BindingDB Entry DOI: 10.7270/Q2BV7GV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181038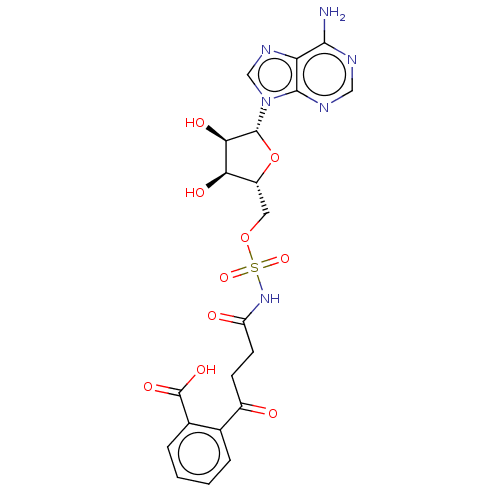 (OSB-AMS (1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Mus musculus) | BDBM50325986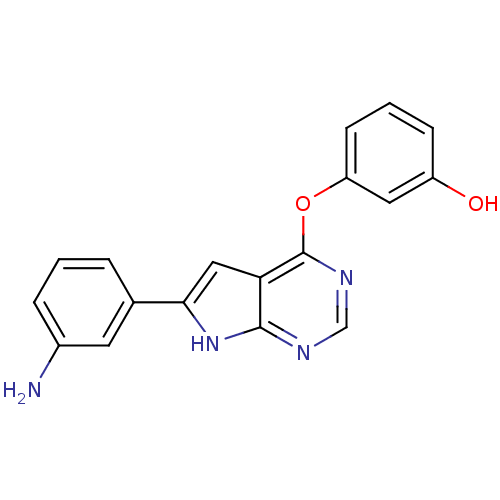 (3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Binding affinity to GSK-3 beta in mouse D3 cells | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50339905 (5'-O-(N-(L-CYSTEINYL)-SULFAMOYL)ADENOSINE | 5'-O-[...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181041 (5'-O-(N-[4-(2-(2-Methoxy-3,4-dioxocyclobut-1-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197294 (CHEMBL3897138) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197291 (AlaSA) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181048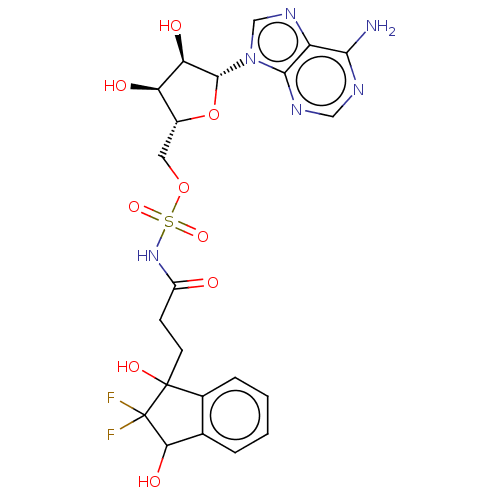 (5'-O-(N-[3-(2,2-Difluoro-1-hydroxy-3-oxo-2,3-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181040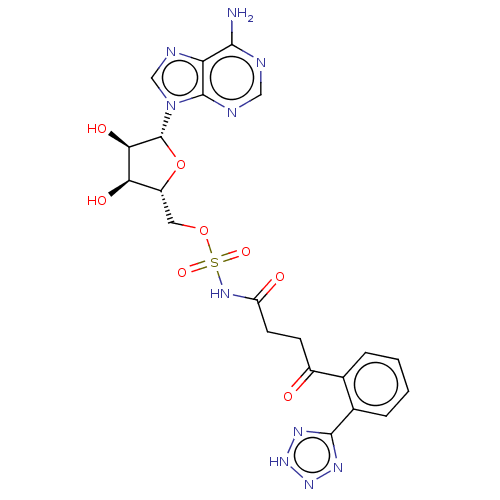 (5'-O-(N-[4-(2-(2H-Tetrazol-5-yl)phenyl)-4-oxob...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197295 (CHEMBL3934723) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181046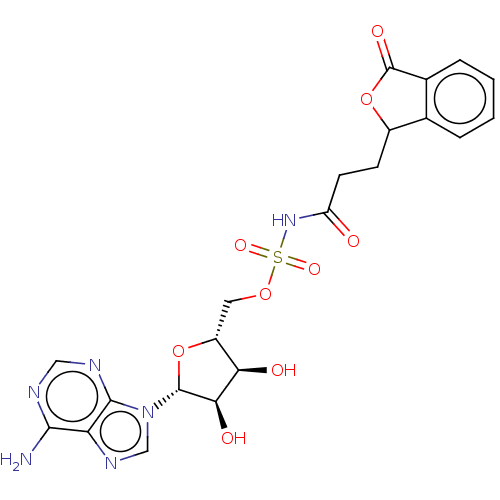 (5'-O-(N-[3-(3-Oxo-1,3-dihydroisobenzofuran-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181045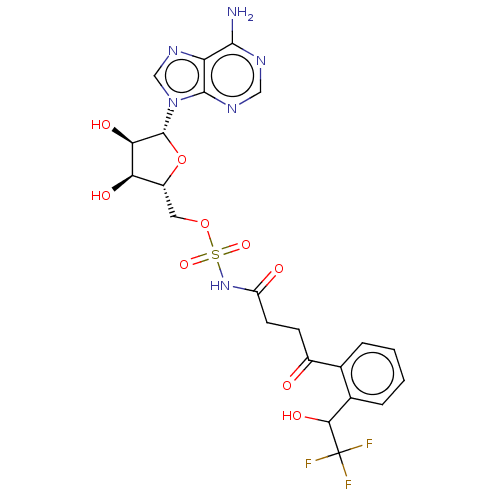 (5'-O-(N-[4-Hydroxy-4-(2-(2,2,2-trifluoro-1-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181044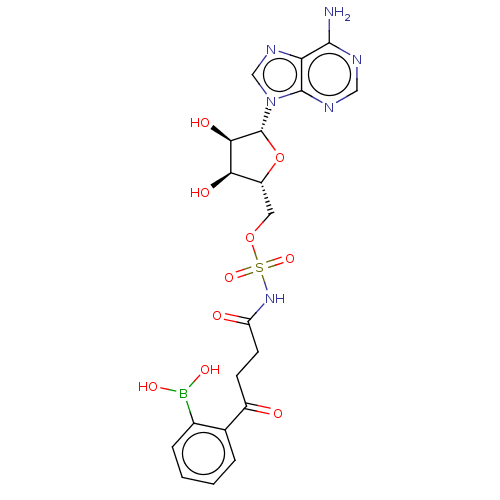 (5'-O-(N-[4-(2-Boronophenyl)-4-oxobutanoyl]sulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181043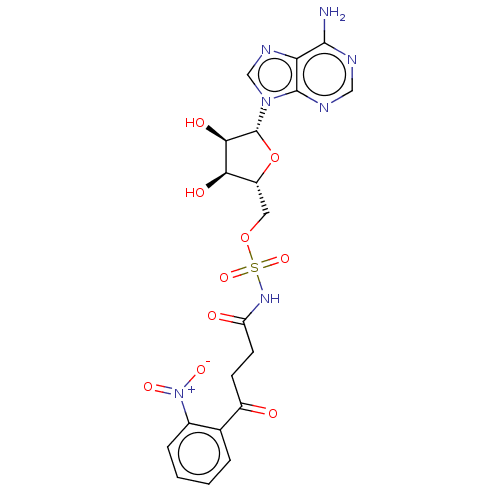 (5'-O-(N-[4-(2-Nitrophenyl)-4-oxobutanoyl]sulfa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181042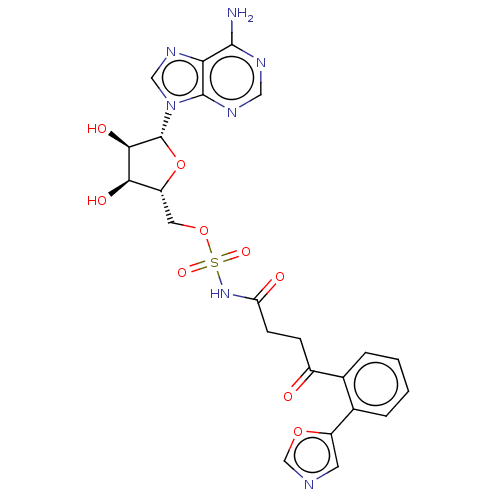 (5'-O-(N-[4-(2-(5-Oxazolyl)phenyl)-4-oxobutanoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181047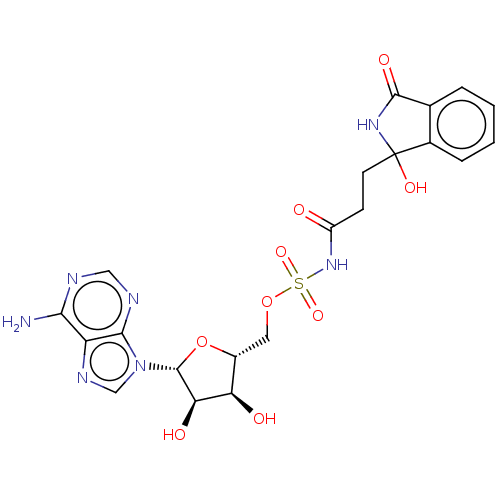 (5'-O-(N-[3-(1-Hydroxy-3-oxoisoindolin-1-yl)pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| o-succinylbenzoate synthase (Escherichia coli K-12 (Enterobacteria)) | BDBM181039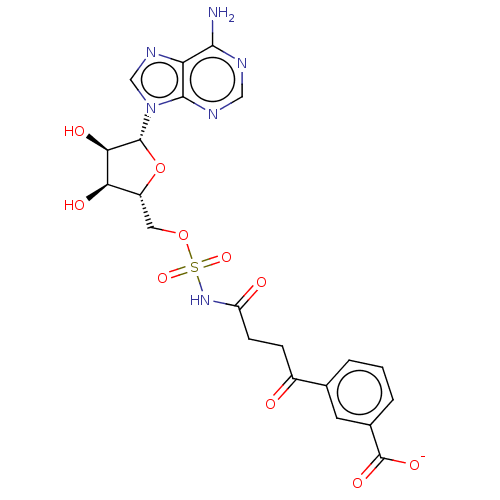 (5'-O-(N-[4''-(3-carboxylphenyl)-4''-oxobutanoyl]su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Argonne National Laboratory | Assay Description Enzyme inhibition studies were performed in 20 mM NaHPO4 buffer (pH 7.4) containing 150 mM NaCl and 1 mM MgCl2 using a MenE-MenB coupled assay in whi... | Biochemistry 54: 6514-24 (2015) Article DOI: 10.1021/acs.biochem.5b00966 BindingDB Entry DOI: 10.7270/Q2RX99WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197292 (CHEMBL3889888) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin biosynthetic protein (Yersinia pestis) | BDBM50197293 (CHEMBL3968275) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Graduate School of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Yersinia pestis C-terminal His6-tagged HMWP2 cysteine adenylation domain (1 to 1491 residues) expressed in Escherichia coli... | Bioorg Med Chem Lett 26: 5340-5345 (2016) Article DOI: 10.1016/j.bmcl.2016.09.027 BindingDB Entry DOI: 10.7270/Q2CZ394B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Mus musculus) | BDBM50325986 (3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Inhibition of GSK-3 beta in mouse D3 cells assessed as induction of neuron specific marker beta3-tubulin by immunofluorescence method | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Mus musculus) | BDBM50325986 (3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Inhibition of GSK-3 beta in mouse D3 cells assessed as induction of neuron specific marker neurofilament-M by immunofluorescence method | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Mus musculus) | BDBM50325986 (3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Inhibition of GSK-3 beta in mouse D3 cells | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM22449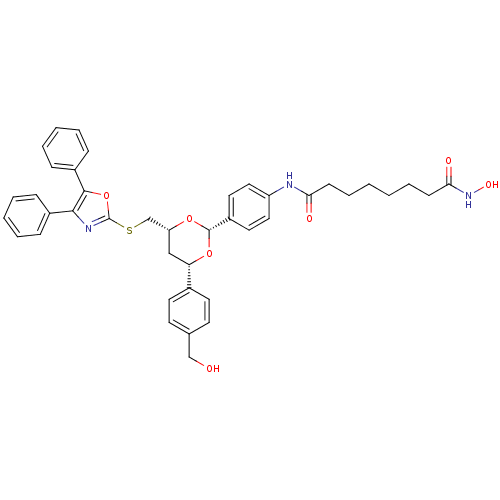 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Inhibition of HDAC6 in human A549 cells assessed as induction of alpha-tubulin acetylation by fluorescence microscopy | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50167161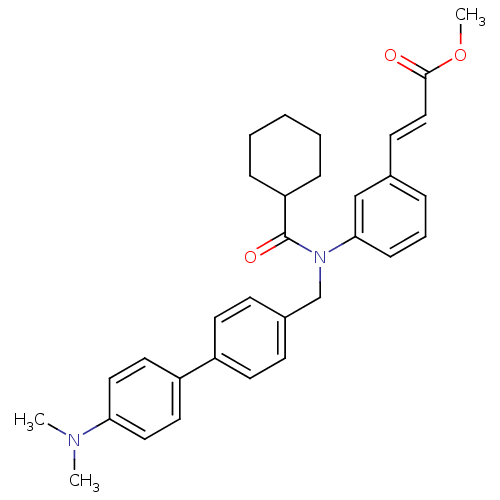 ((E)-3-{3-[Cyclohexanecarbonyl-(4'-dimethylamino-bi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Agonist activity at FXR | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Mus musculus) | BDBM50325986 (3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by ChEMBL | Assay Description Inhibition of GSK-3 beta in mouse D3 cells assessed as induction of neuron specific marker neurofilament-M by immunofluorescence method | Nat Chem Biol 1: 74-84 (2006) Article DOI: 10.1038/nchembio0705-74 BindingDB Entry DOI: 10.7270/Q2MC9118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||