

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50291312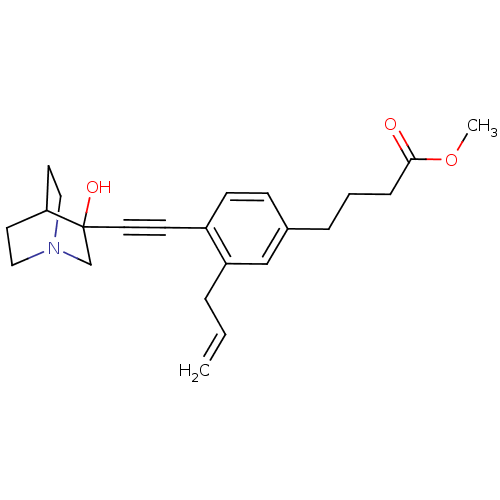 (4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719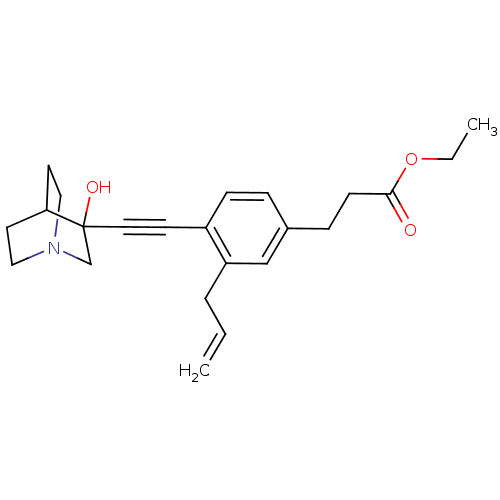 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291315 (5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291311 (6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291316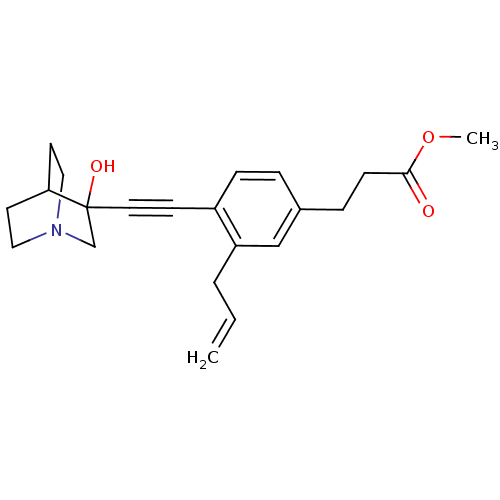 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291317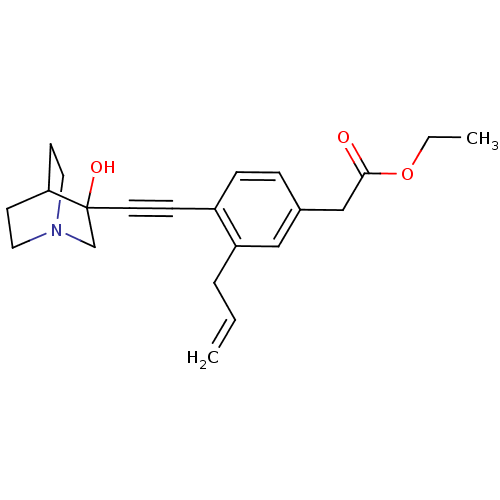 (CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291314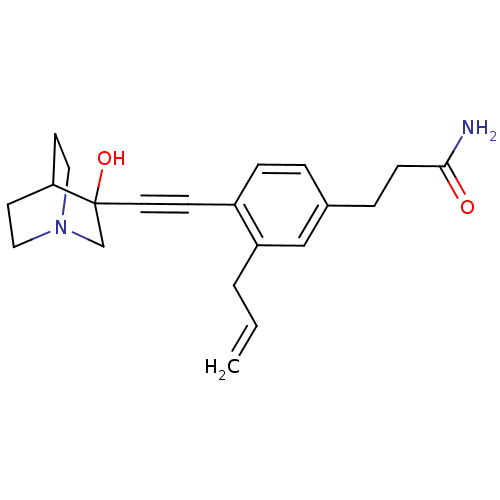 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291319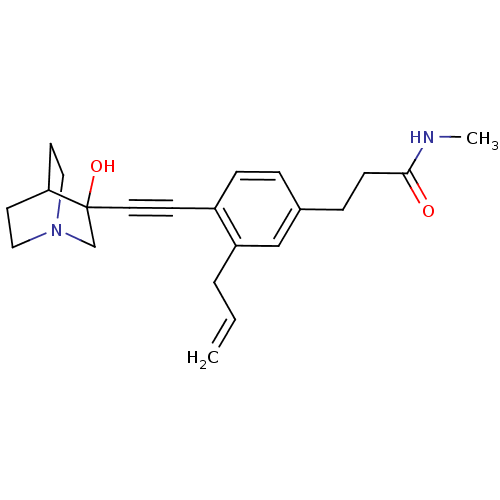 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291318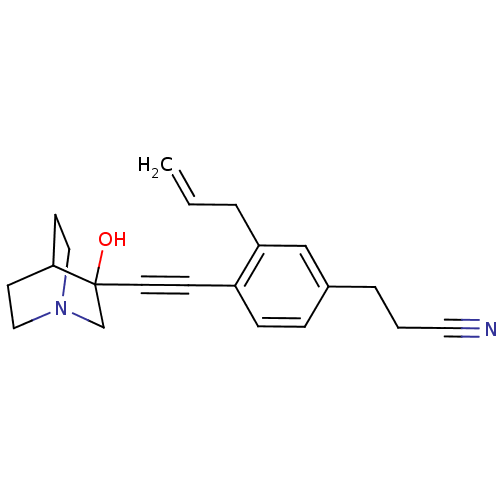 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291313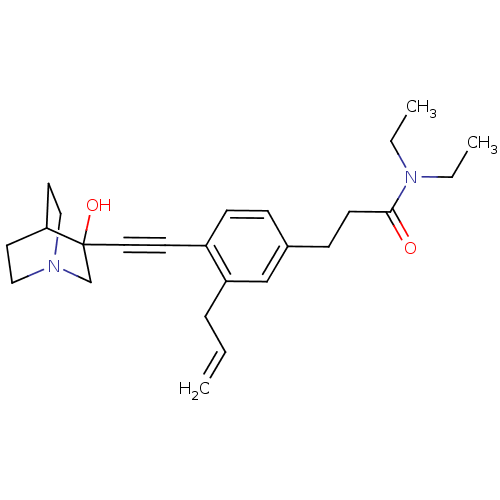 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047126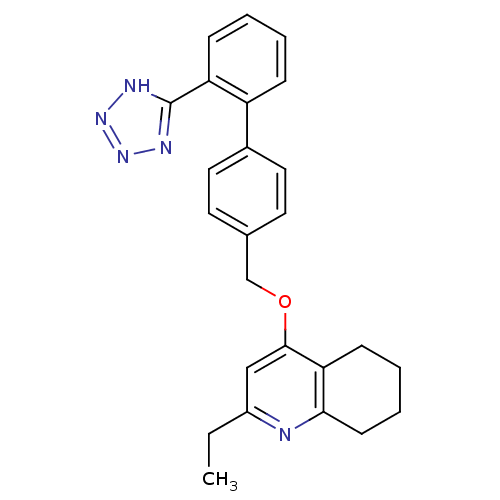 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283596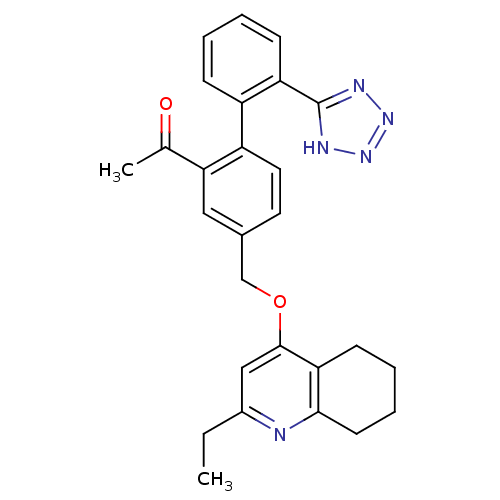 (1-[4-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxym...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283591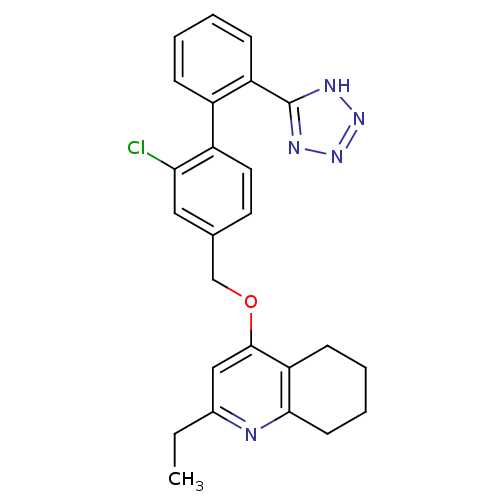 (4-[2-Chloro-2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283597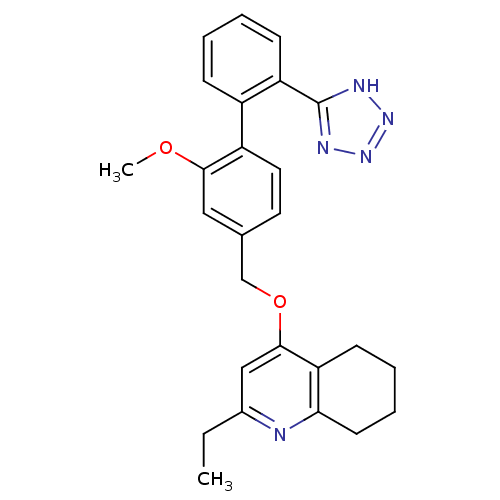 (2-Ethyl-4-[2-methoxy-2'-(2H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283587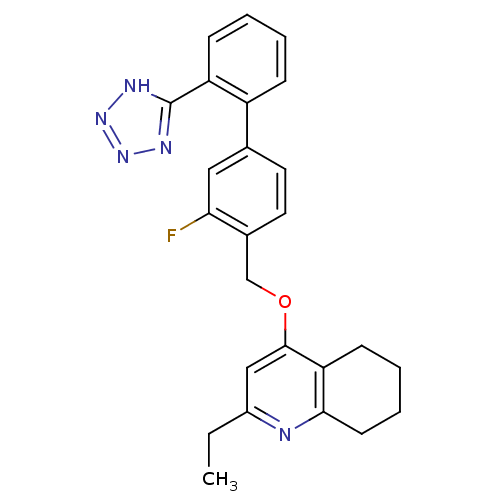 (2-Ethyl-4-[3-fluoro-2'-(2H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50009714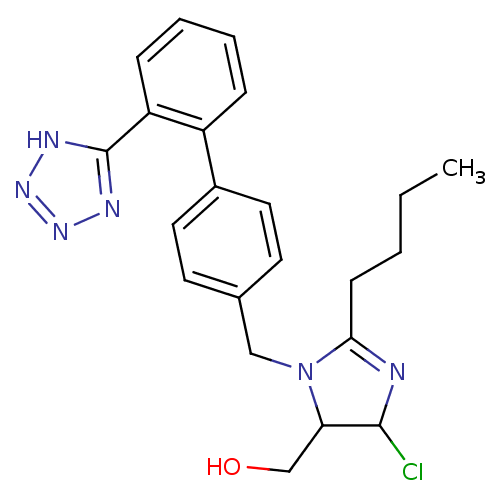 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283593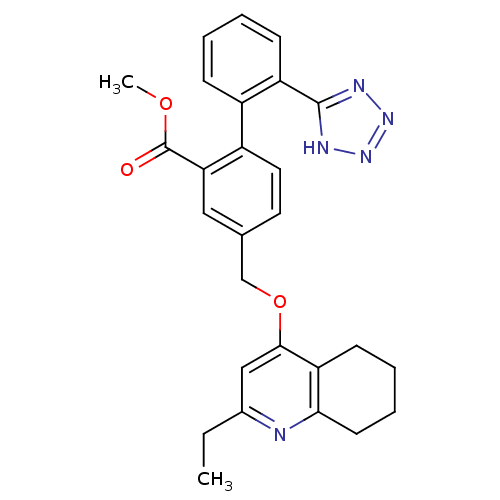 (4-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283590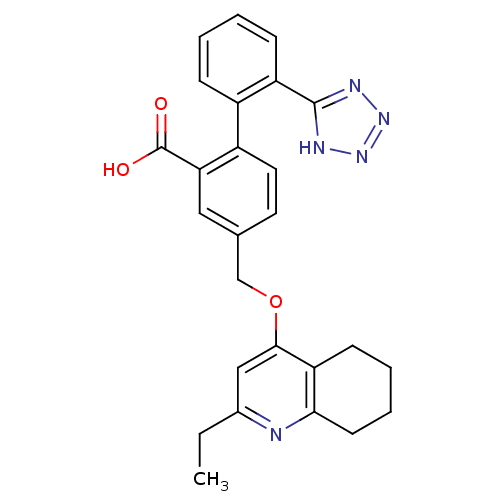 (4-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283589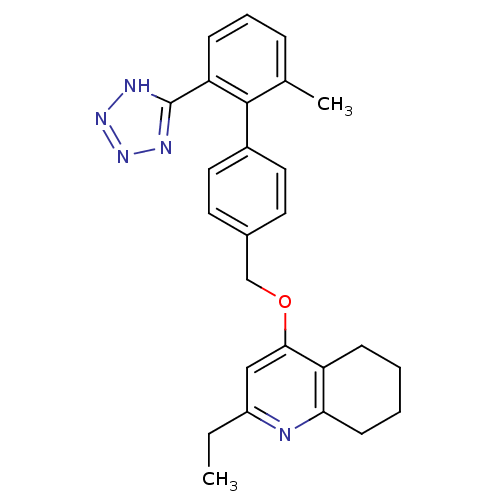 (2-Ethyl-4-[2'-methyl-6'-(2H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283592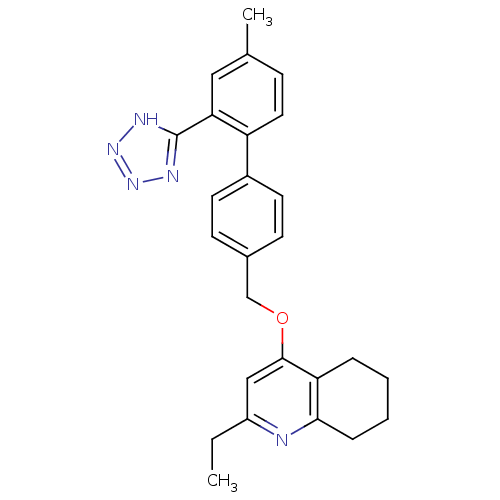 (2-Ethyl-4-[4'-methyl-2'-(2H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283595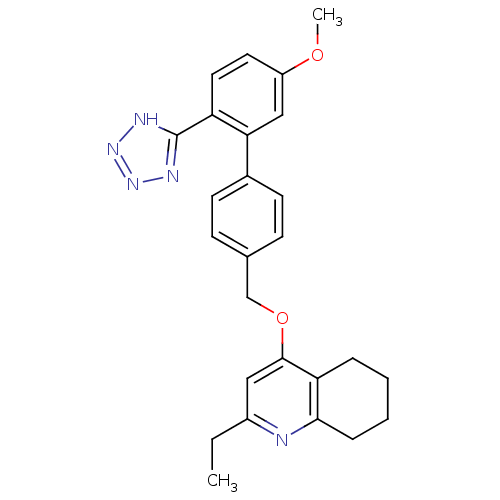 (2-Ethyl-4-[5'-methoxy-2'-(2H-tetrazol-5-yl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283588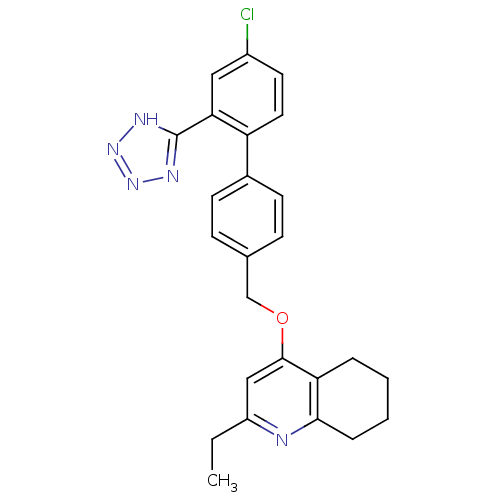 (4-[4'-Chloro-2'-(2H-tetrazol-5-yl)-biphenyl-4-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50005340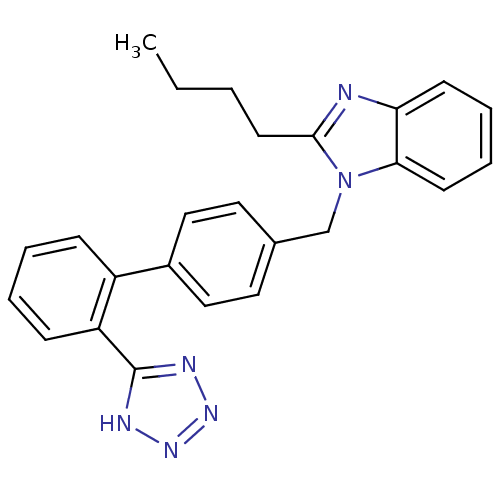 (2-Butyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283594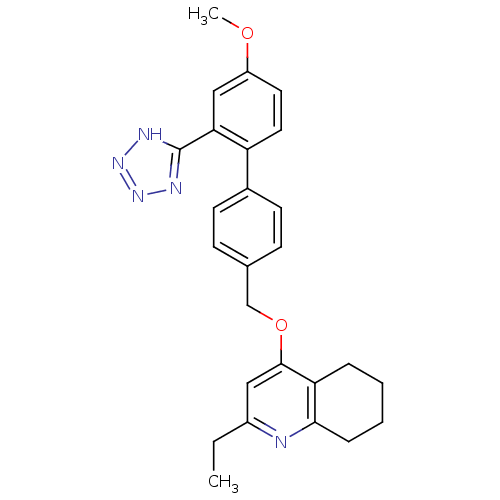 (2-Ethyl-4-[4'-methoxy-2'-(2H-tetrazol-5-yl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50003399 (4'-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230428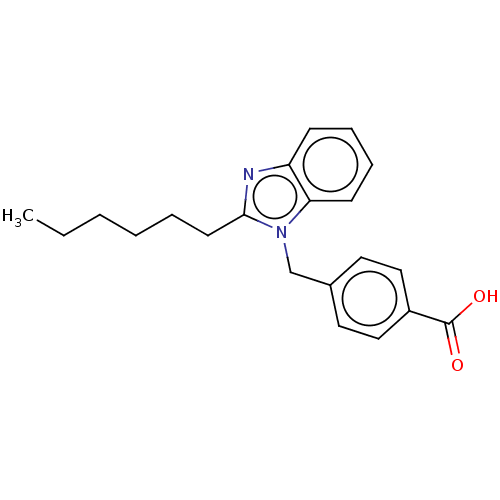 (CHEMBL352376) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230436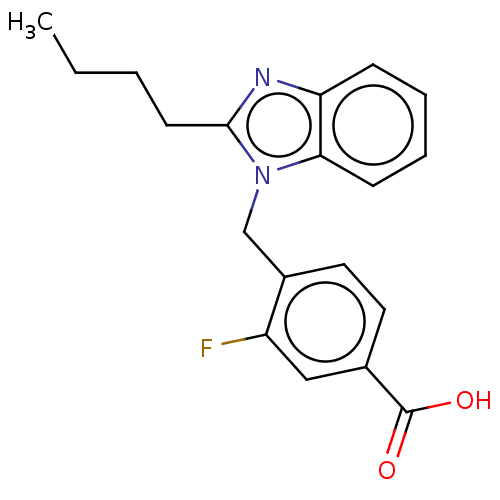 (CHEMBL424372) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230438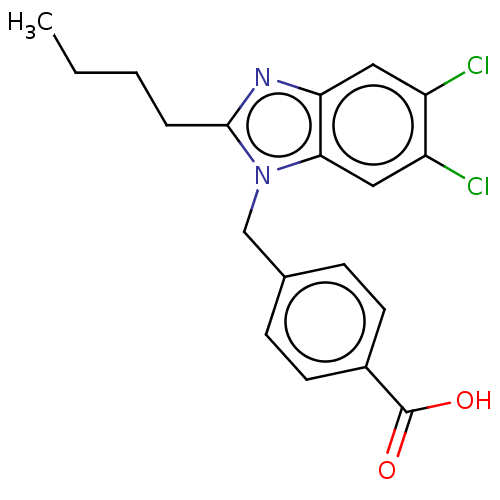 (CHEMBL171530) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230431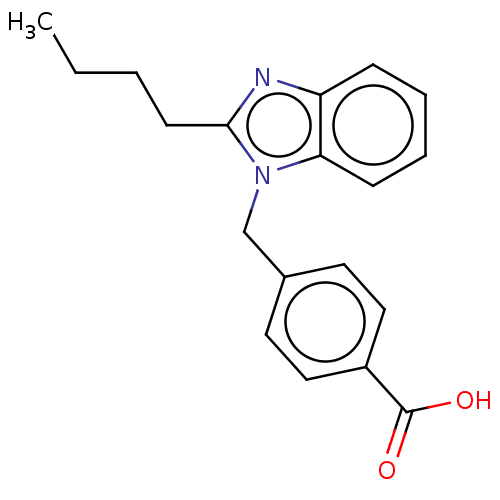 (CHEMBL171456) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230439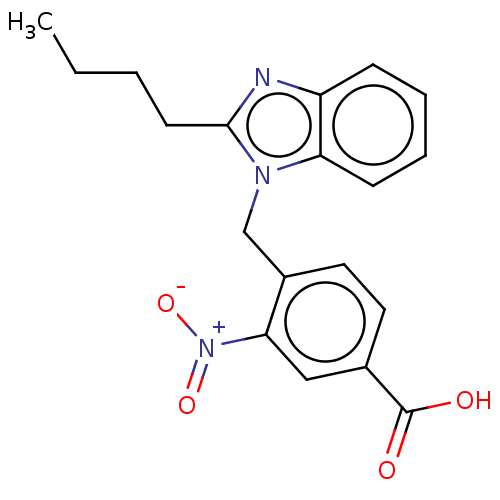 (CHEMBL172344) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50005347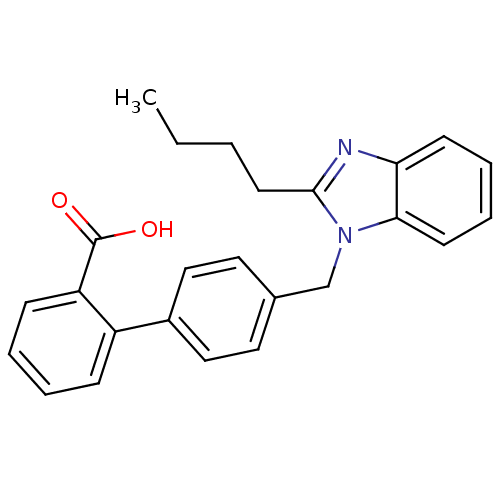 (4'-((2-butyl-1H-benzo[d]imidazol-1-yl)methyl)biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230427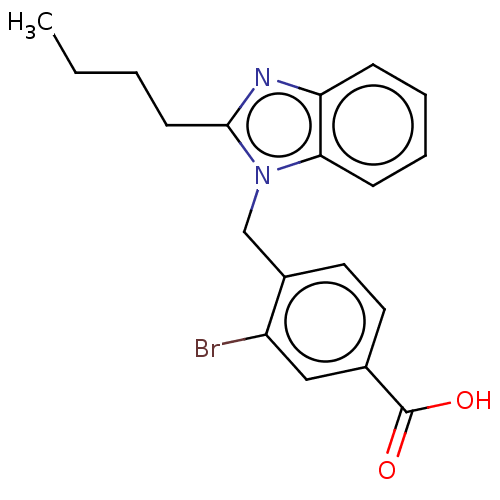 (CHEMBL171490) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230440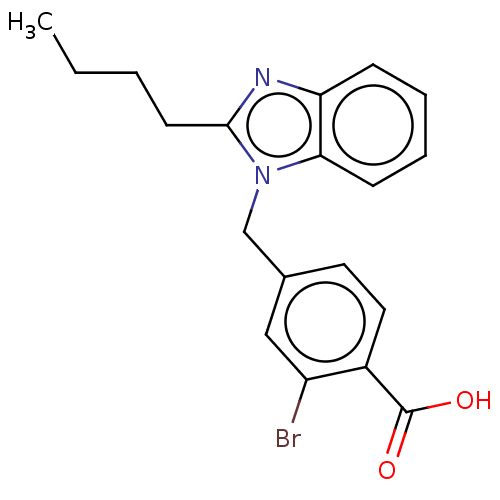 (CHEMBL171395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230435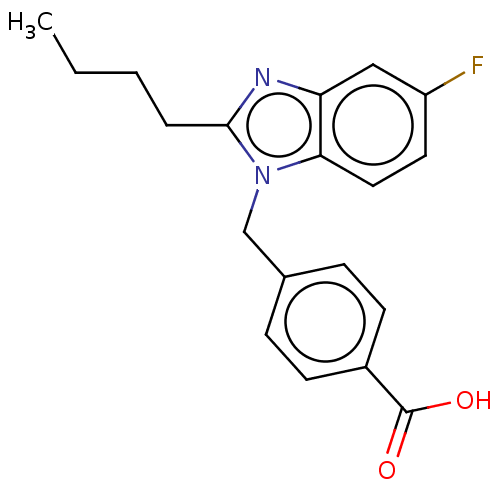 (CHEMBL172577) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230434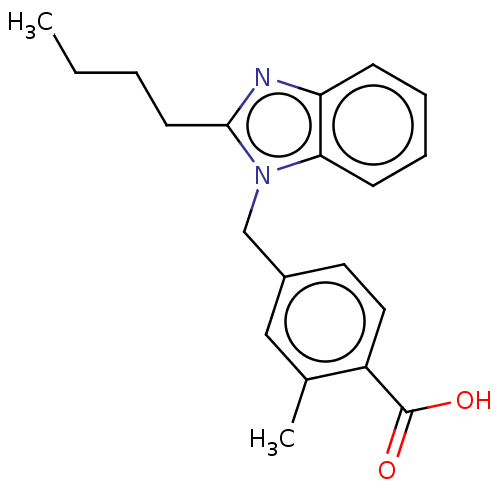 (CHEMBL172056) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230424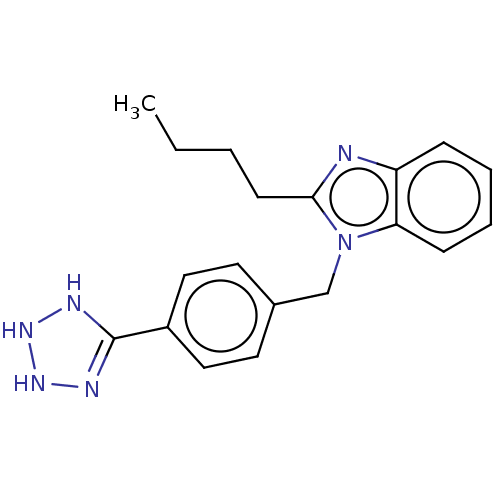 (CHEMBL558388) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230429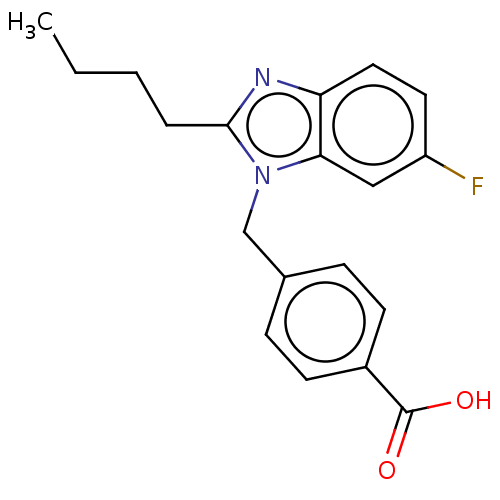 (CHEMBL354435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230425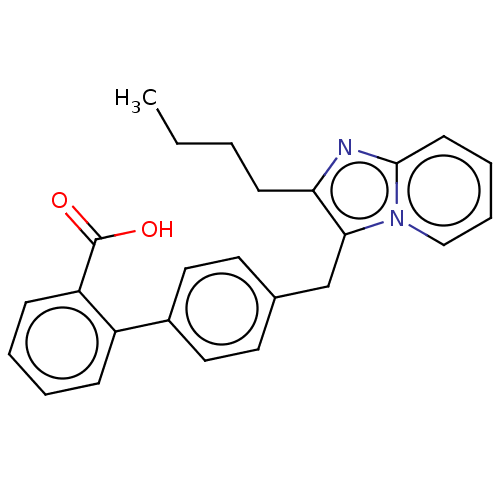 (CHEMBL355691) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230426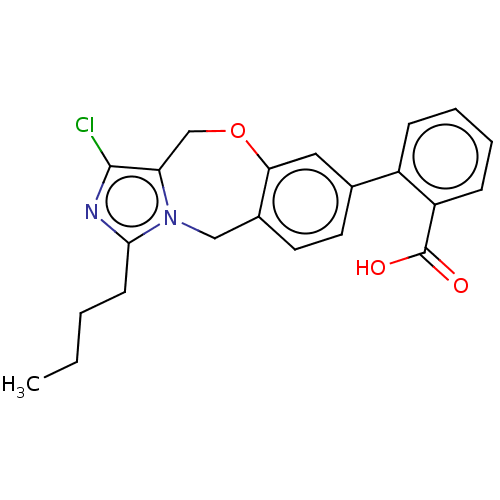 (CHEMBL367565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230441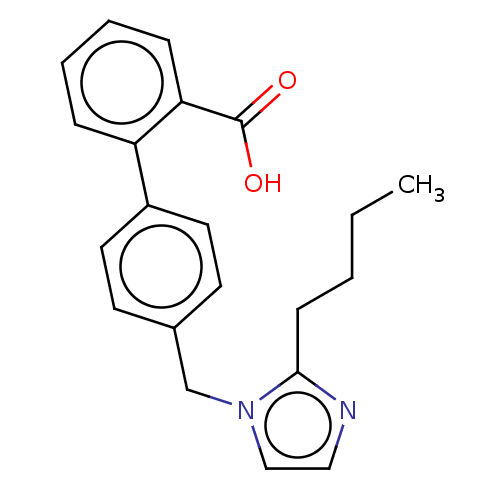 (CHEMBL171681) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230539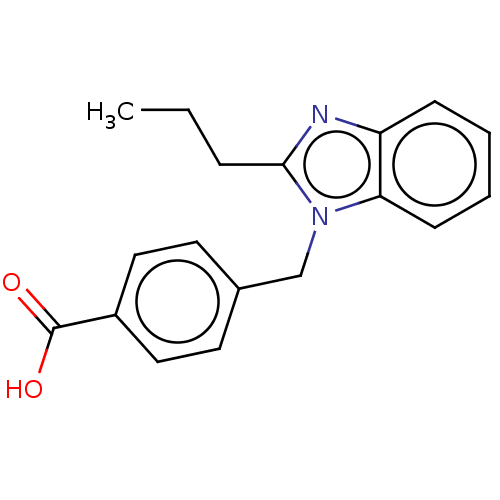 (CHEMBL172334) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230437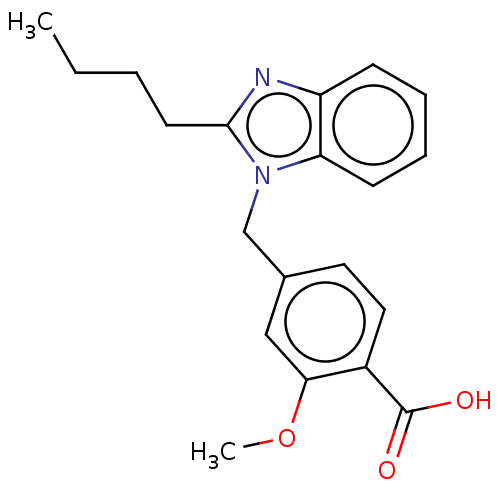 (CHEMBL367566) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230430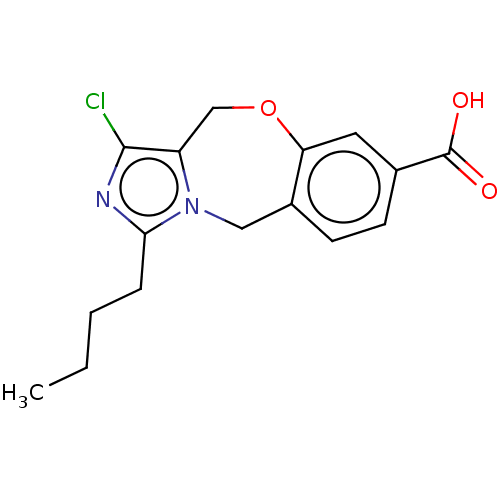 (CHEMBL354436) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230432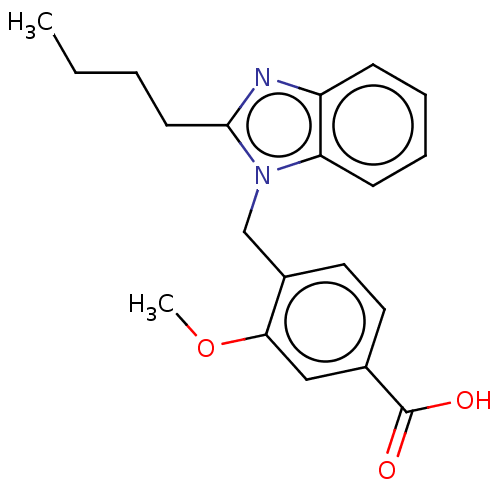 (CHEMBL171317) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230423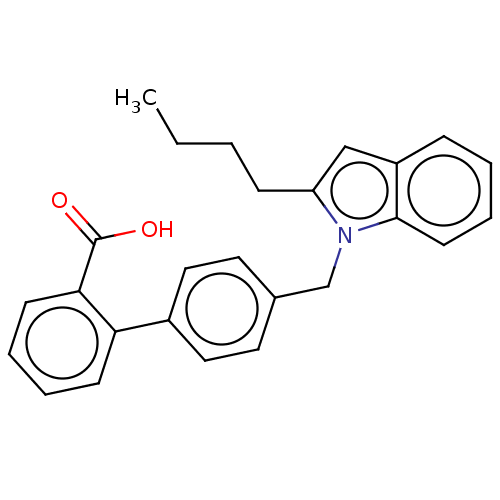 (CHEMBL171875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50230433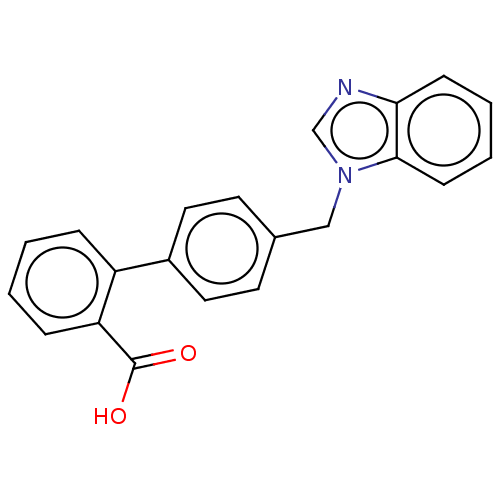 (CHEMBL422562) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3-[125I]-iodotyrosyl]-angiotensin II binding to Angiotensin II receptors in the membrane preparations of guinea pig adrenal glands. | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||