

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135773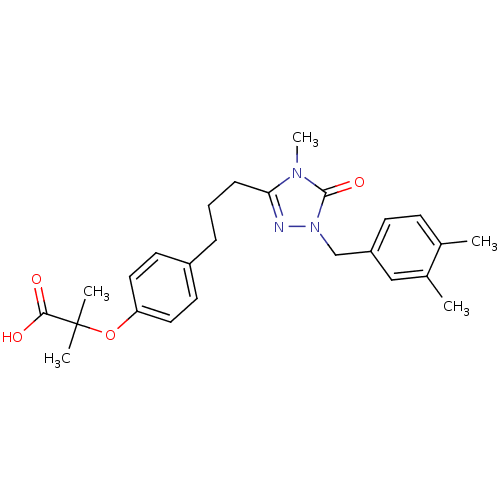 (2-(4-{3-[1-(3,4-Dimethyl-benzyl)-4-methyl-5-oxo-4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135777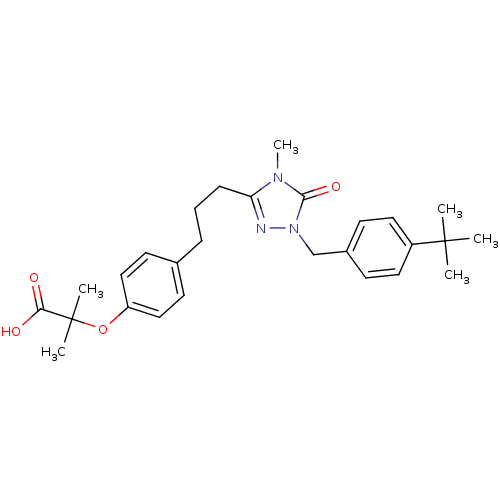 (2-(4-{3-[1-(4-tert-Butyl-benzyl)-4-methyl-5-oxo-4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135775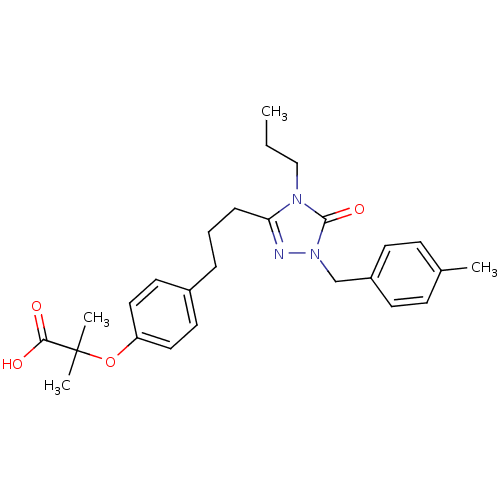 (2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135782 (2-(4-{3-[1-(3-Methoxy-benzyl)-5-oxo-4-propyl-4,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135780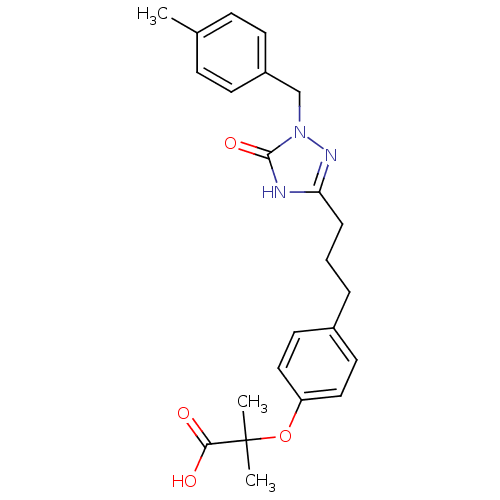 (2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135779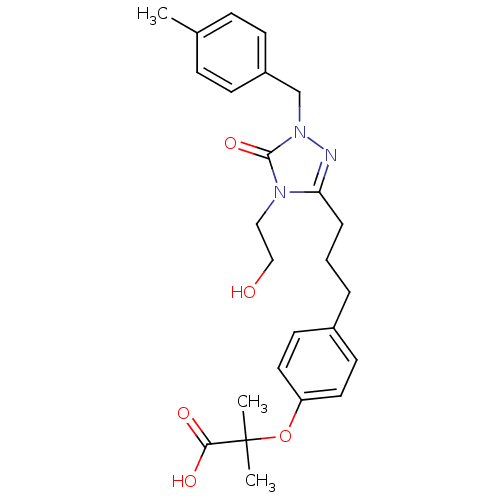 (2-(4-{3-[4-(2-Hydroxy-ethyl)-1-(4-methyl-benzyl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28799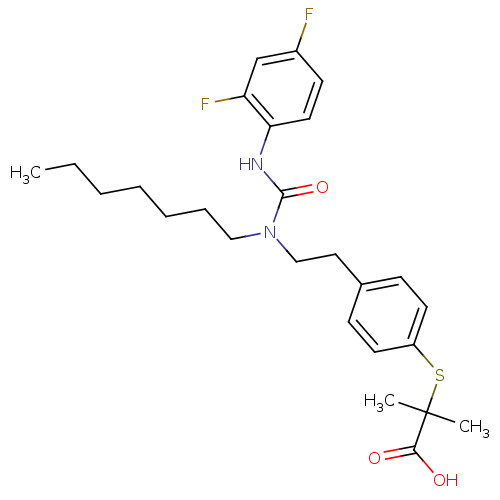 (2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135781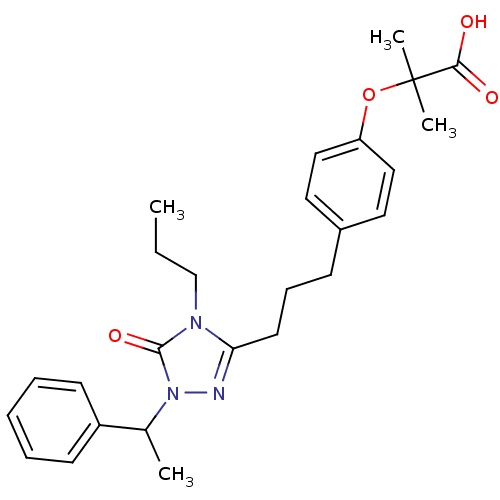 (2-Methyl-2-(4-{3-[5-oxo-1-(1-phenyl-ethyl)-4-propy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135774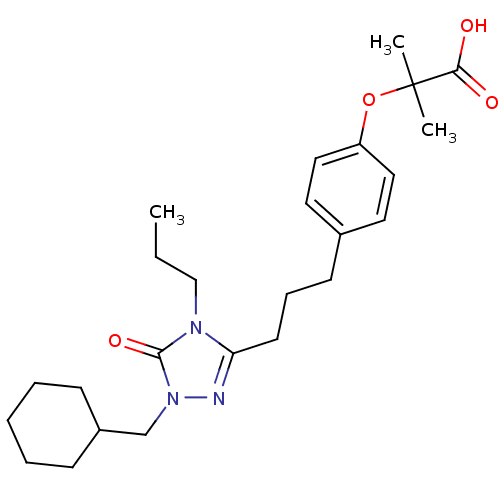 (2-{4-[3-(1-Cyclohexylmethyl-5-oxo-4-propyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490735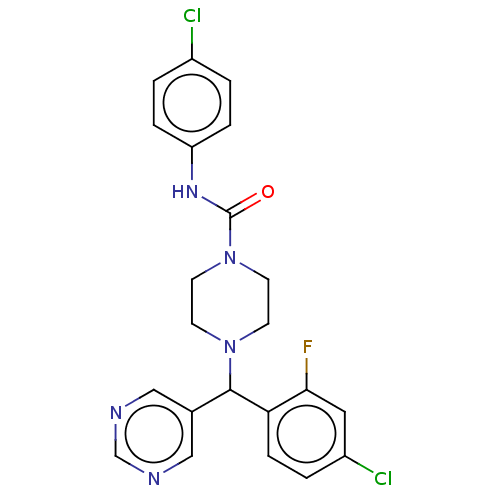 (CHEMBL1863002 | DNDI1449875) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135777 (2-(4-{3-[1-(4-tert-Butyl-benzyl)-4-methyl-5-oxo-4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28799 (2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490750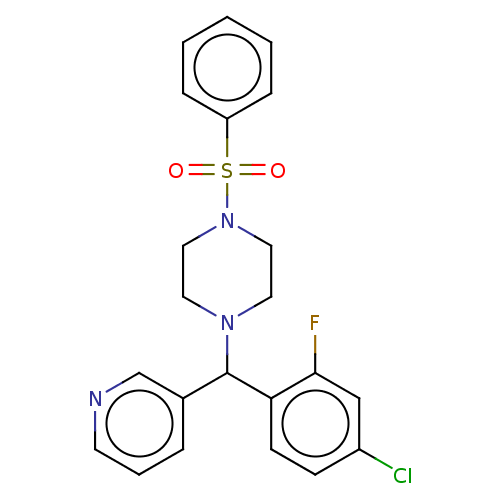 (CHEMBL2334336) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135773 (2-(4-{3-[1-(3,4-Dimethyl-benzyl)-4-methyl-5-oxo-4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490748 (CHEMBL1863056 | DNDI1435775) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135774 (2-{4-[3-(1-Cyclohexylmethyl-5-oxo-4-propyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50135776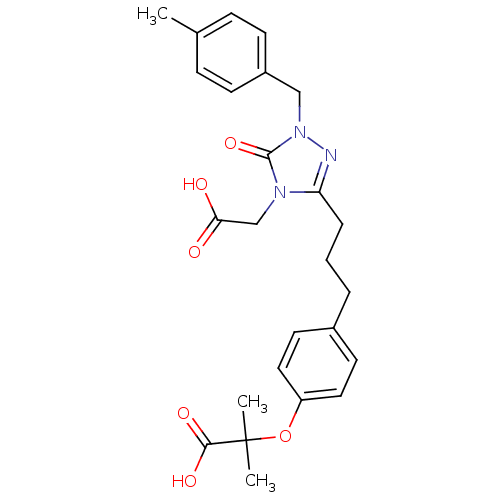 (2-(4-{3-[4-Carboxymethyl-1-(4-methyl-benzyl)-5-oxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490744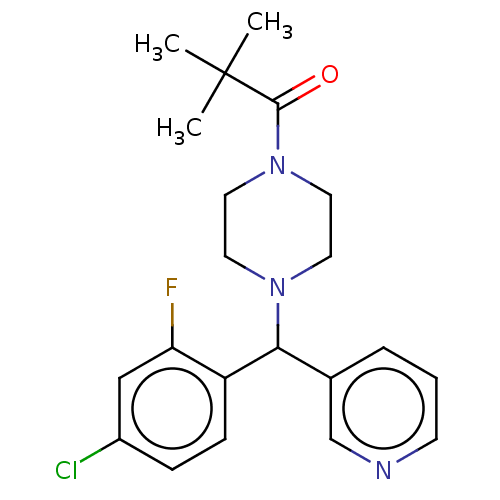 (CHEMBL1862905 | DNDI1467768) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490737 (CHEMBL1863288 | DNDI1538112) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135775 (2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490736 (CHEMBL1863145 | DNDI1343536) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135781 (2-Methyl-2-(4-{3-[5-oxo-1-(1-phenyl-ethyl)-4-propy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135777 (2-(4-{3-[1-(4-tert-Butyl-benzyl)-4-methyl-5-oxo-4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135773 (2-(4-{3-[1-(3,4-Dimethyl-benzyl)-4-methyl-5-oxo-4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135774 (2-{4-[3-(1-Cyclohexylmethyl-5-oxo-4-propyl-4,5-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490742 (CHEMBL1863462 | DNDI1460901) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490749 (CHEMBL1862798 | DNDI1434923) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490743 (CHEMBL1863046 | DNDI1434940) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135782 (2-(4-{3-[1-(3-Methoxy-benzyl)-5-oxo-4-propyl-4,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490753 (CHEMBL1863455 | DNDI1465083) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490741 (CHEMBL2334337) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490751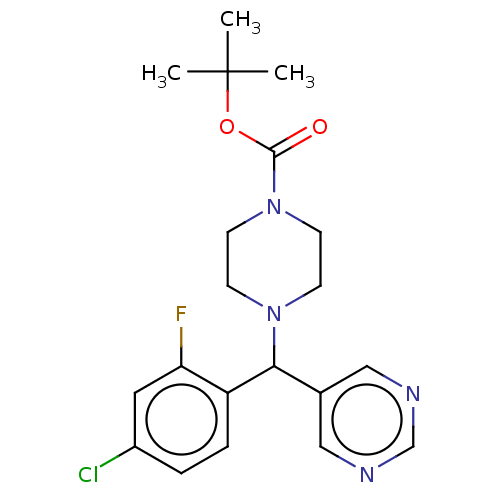 (CHEMBL1863306 | DNDI1434944) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490745 (CHEMBL1863273 | DNDI1338491) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135780 (2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490746 (CHEMBL1863393 | DNDI1388149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490752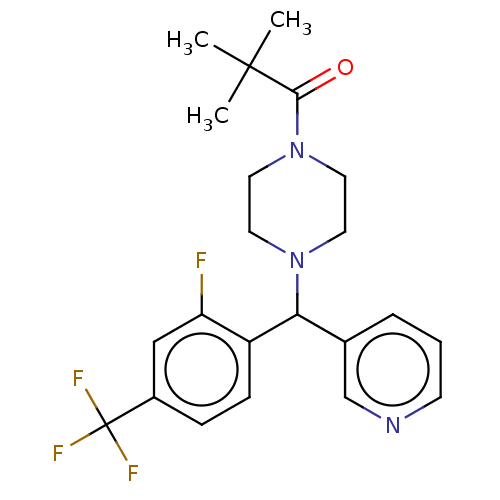 (CHEMBL1862941 | DNDI1551253) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50135779 (2-(4-{3-[4-(2-Hydroxy-ethyl)-1-(4-methyl-benzyl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor delta was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135775 (2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4-prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490738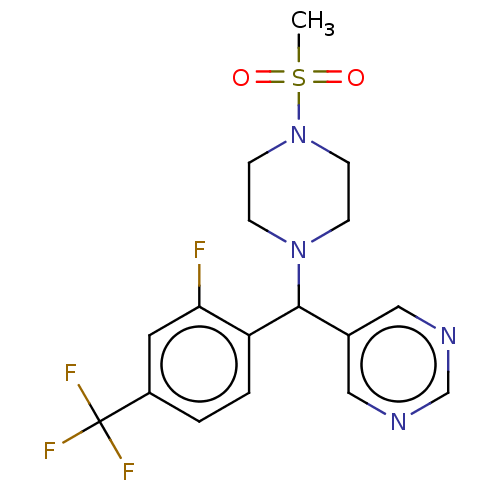 (CHEMBL1863131 | DNDI1490391) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135781 (2-Methyl-2-(4-{3-[5-oxo-1-(1-phenyl-ethyl)-4-propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135779 (2-(4-{3-[4-(2-Hydroxy-ethyl)-1-(4-methyl-benzyl)-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135782 (2-(4-{3-[1-(3-Methoxy-benzyl)-5-oxo-4-propyl-4,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135780 (2-Methyl-2-(4-{3-[1-(4-methyl-benzyl)-5-oxo-4,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490747 (CHEMBL1863090 | DNDI1449876) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490740 (CHEMBL1862938 | DNDI1467767) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50490739 (CHEMBL1862975 | DNDI1335908) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes assessed as reduction in testosterone-beta-hydroxylation | Bioorg Med Chem 21: 1756-63 (2013) Article DOI: 10.1016/j.bmc.2013.01.050 BindingDB Entry DOI: 10.7270/Q2WH2SWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50135776 (2-(4-{3-[4-Carboxymethyl-1-(4-methyl-benzyl)-5-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determined | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50266776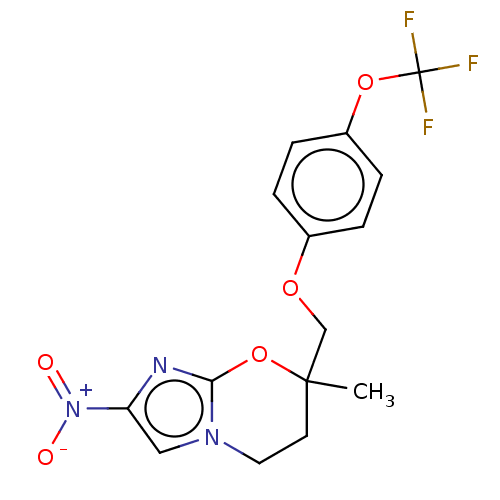 (CHEMBL4066553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsome using testosterone as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112849 BindingDB Entry DOI: 10.7270/Q2J67MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50556552 (CHEMBL583256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsome using testosterone as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112849 BindingDB Entry DOI: 10.7270/Q2J67MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28700 (2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Eli Lilly & Company Curated by ChEMBL | Assay Description Inhibitory activity against human Peroxisome proliferator activated receptor alpha | J Med Chem 46: 5121-4 (2003) Article DOI: 10.1021/jm034173l BindingDB Entry DOI: 10.7270/Q2D50MB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |