

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572983 (CHEMBL4848846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572968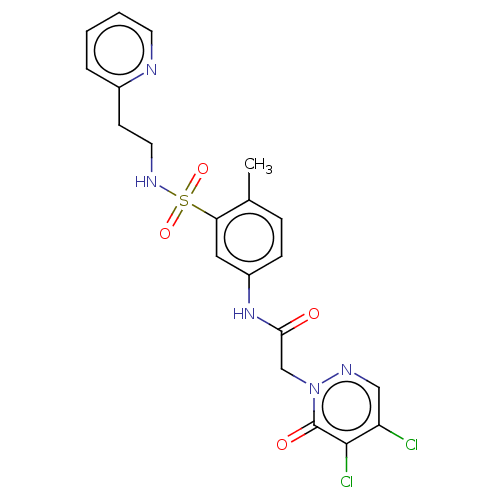 (CHEMBL4862851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572964 (CHEMBL4867592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572989 (CHEMBL4846332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572991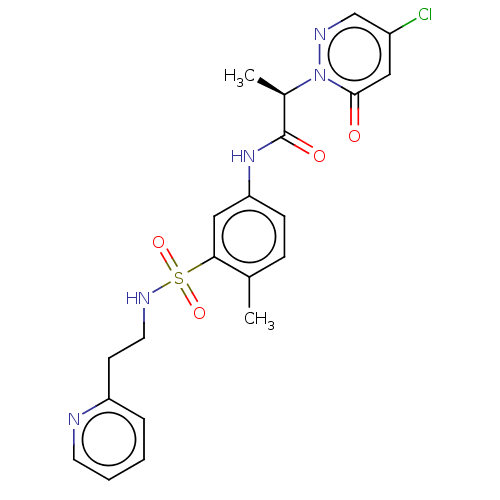 (CHEMBL4858967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572990 (CHEMBL4859105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572988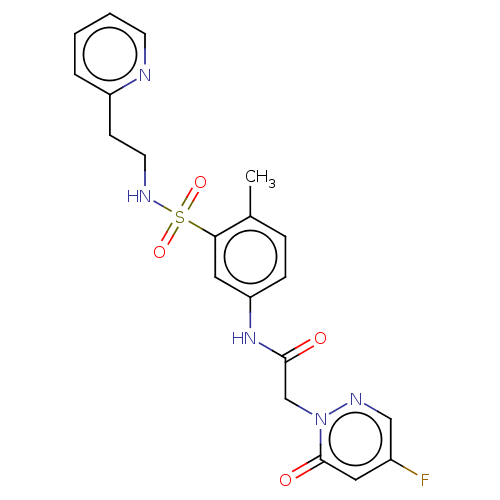 (CHEMBL4855695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572984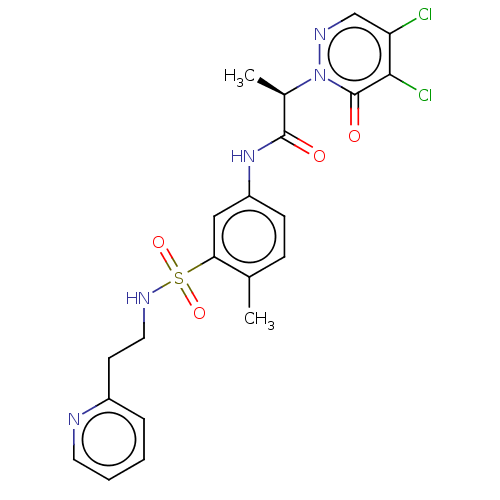 (CHEMBL4874198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM400979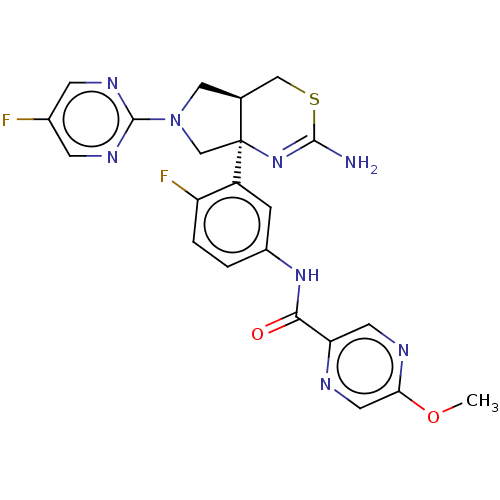 (US9999624, Compound 4) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM150688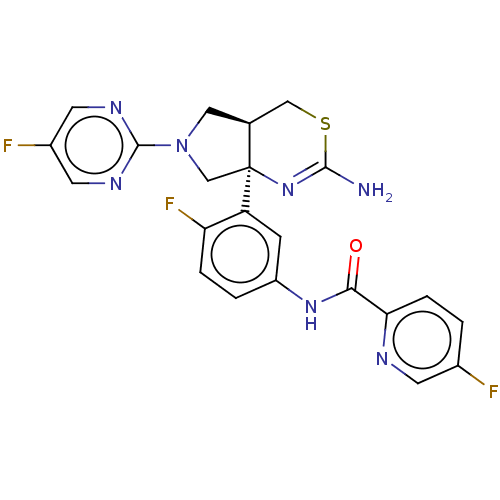 (US8987254, 3 | US9999624, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM150688 (US8987254, 3 | US9999624, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.555 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150688 (US8987254, 3 | US9999624, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM400979 (US9999624, Compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM400979 (US9999624, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012647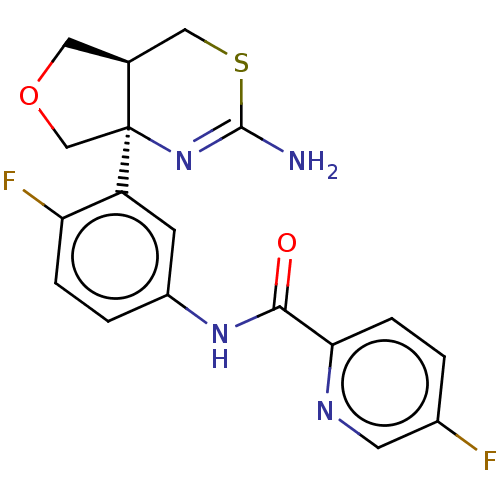 (CHEMBL2396989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15471 (4-[(1-{[2-oxo-2-(thiophen-3-yl)ethyl]amino}cyclope...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 1765-8 (2007) Article DOI: 10.1016/j.bmcl.2006.12.074 BindingDB Entry DOI: 10.7270/Q247483F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50540172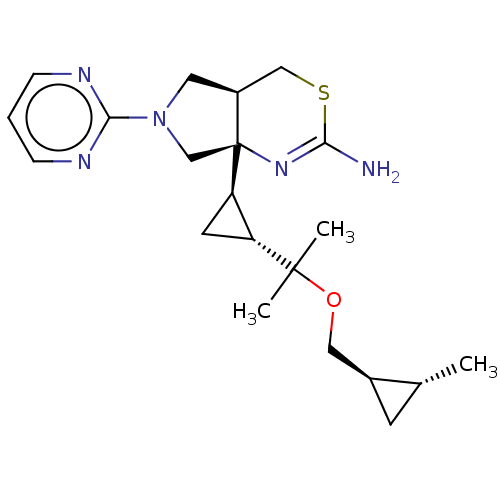 (CHEMBL4637426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540172 (CHEMBL4637426) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012647 (CHEMBL2396989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540172 (CHEMBL4637426) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM50012647 (CHEMBL2396989) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171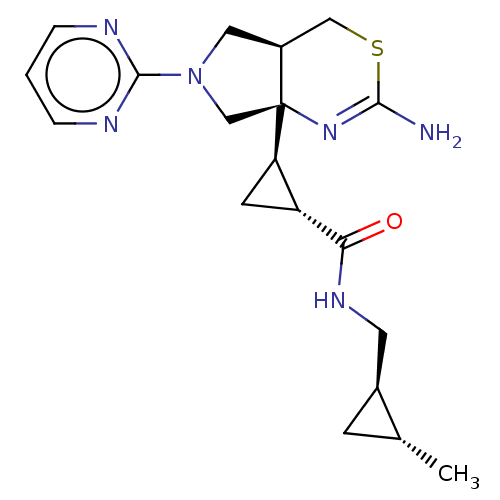 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) by cell based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012647 (CHEMBL2396989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6683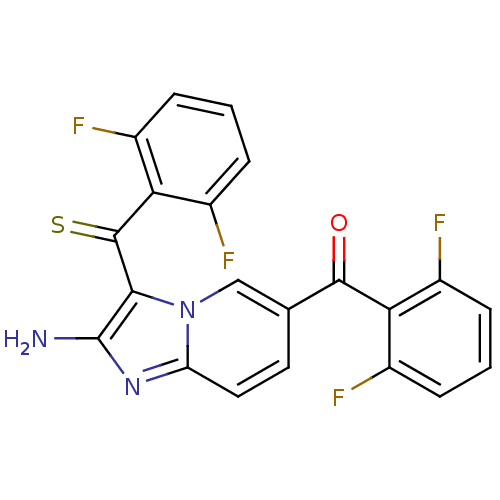 (aminoimidazo[1,2-a]pyridine deriv. 18 | {2-amino-6...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6684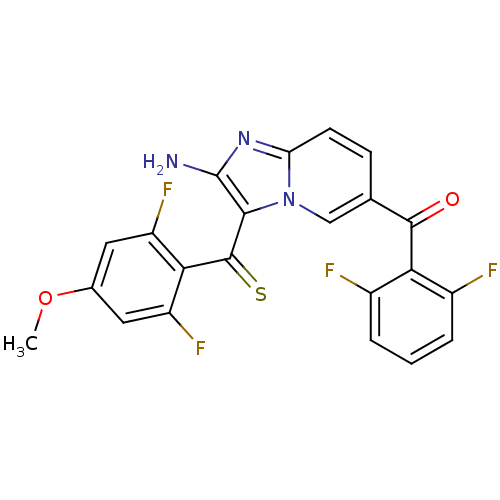 (aminoimidazo[1,2-a]pyridine deriv. 20 | {2-amino-6...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6687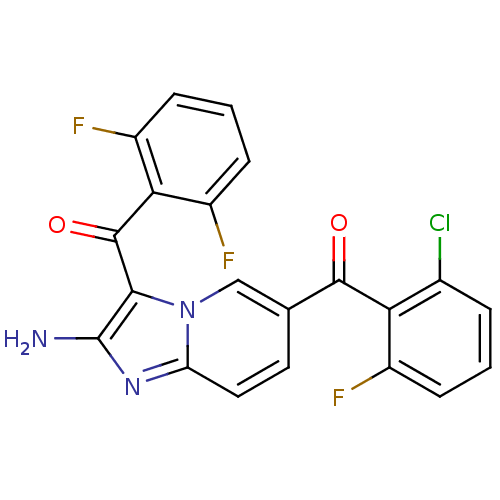 (6-[(2-chloro-6-fluorophenyl)carbonyl]-3-[(2,6-difl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15465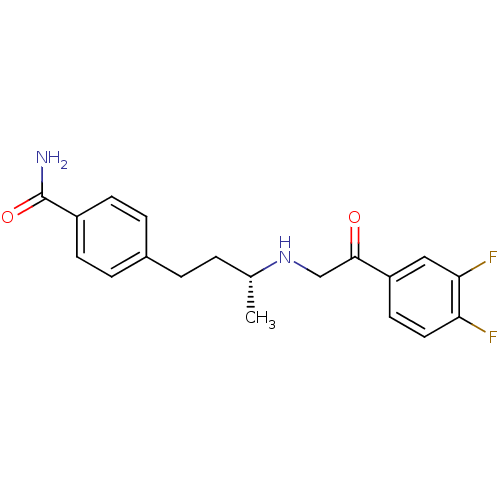 (4-[(3R)-3-{[2-(3,4-difluorophenyl)-2-oxoethyl]amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 1765-8 (2007) Article DOI: 10.1016/j.bmcl.2006.12.074 BindingDB Entry DOI: 10.7270/Q247483F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50540170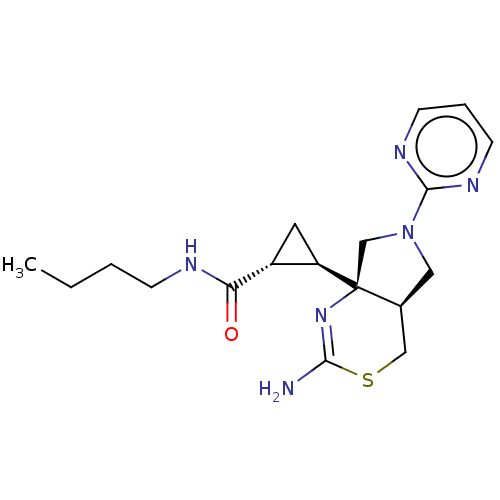 (CHEMBL4636286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6686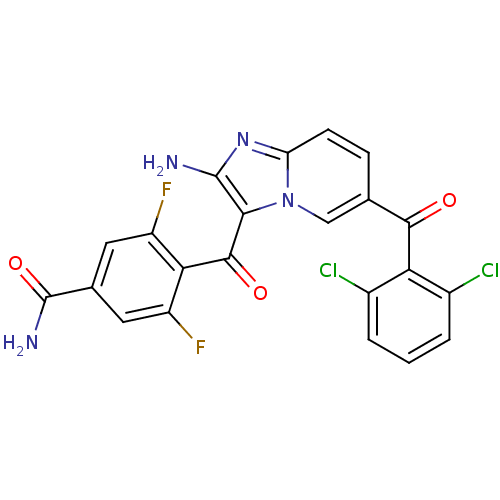 (4-({2-amino-6-[(2,6-dichlorophenyl)carbonyl]imidaz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6702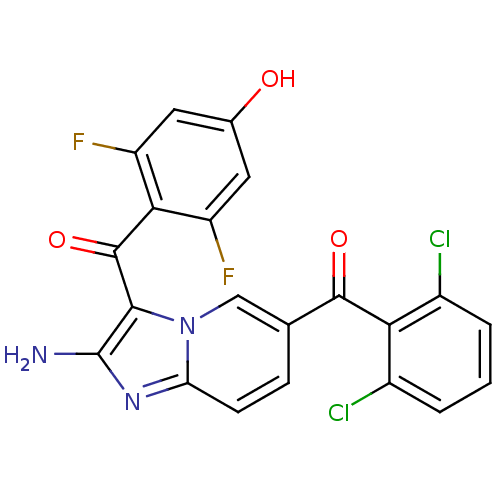 (4-({2-amino-6-[(2,6-dichlorophenyl)carbonyl]imidaz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6697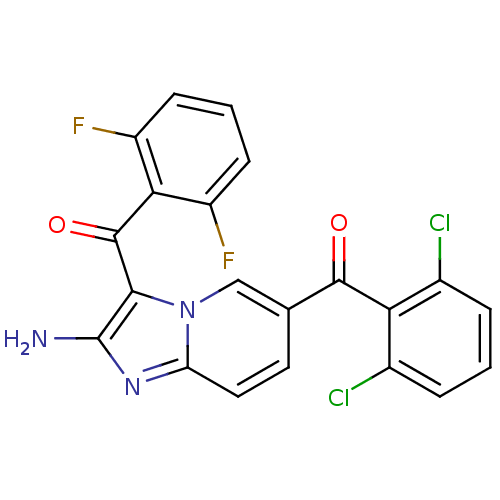 (6-[(2,6-dichlorophenyl)carbonyl]-3-[(2,6-difluorop...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6689 ((2E)-3-{2-amino-3-[(2,6-difluorophenyl)carbonyl]im...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6677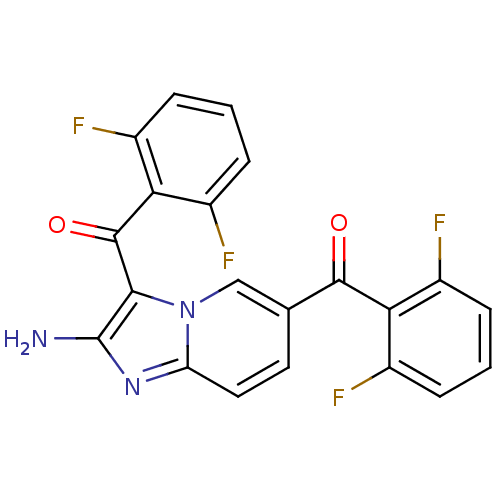 (3,6-bis[(2,6-difluorophenyl)carbonyl]imidazo[1,2-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6688 (3-[(2,6-difluorophenyl)carbonyl]-6-[1-(2,6-difluor...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540170 (CHEMBL4636286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6680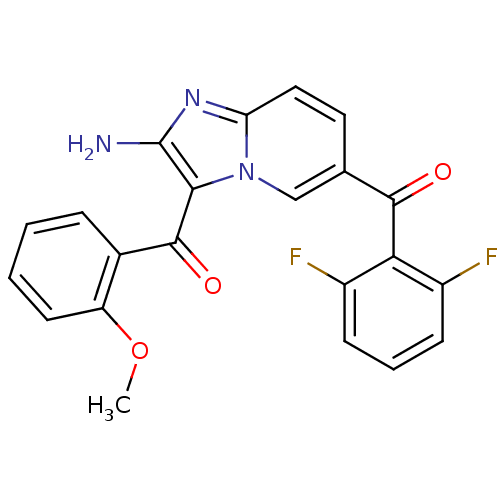 (6-[(2,6-difluorophenyl)carbonyl]-3-[(2-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50581133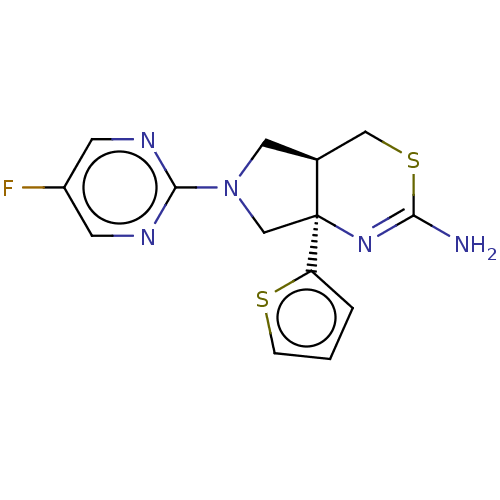 (CHEMBL5093835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15464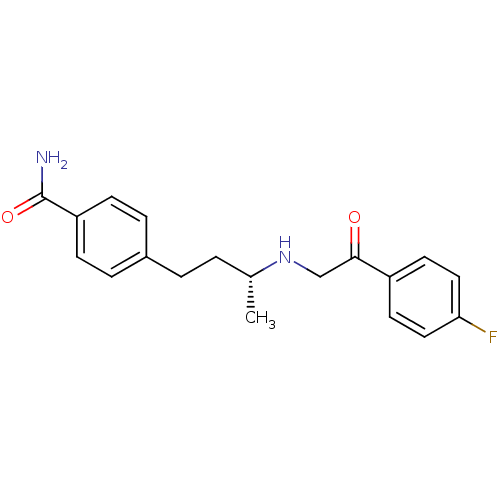 (4-[(3R)-3-{[2-(4-fluorophenyl)-2-oxoethyl]amino}bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 1765-8 (2007) Article DOI: 10.1016/j.bmcl.2006.12.074 BindingDB Entry DOI: 10.7270/Q247483F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6679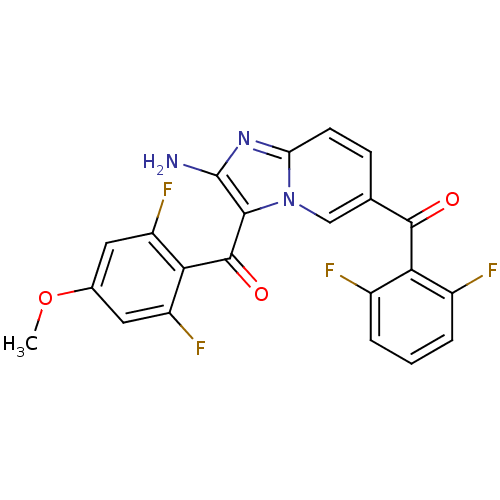 (3-[(2,6-difluoro-4-methoxyphenyl)carbonyl]-6-[(2,6...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6688 (3-[(2,6-difluorophenyl)carbonyl]-6-[1-(2,6-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6685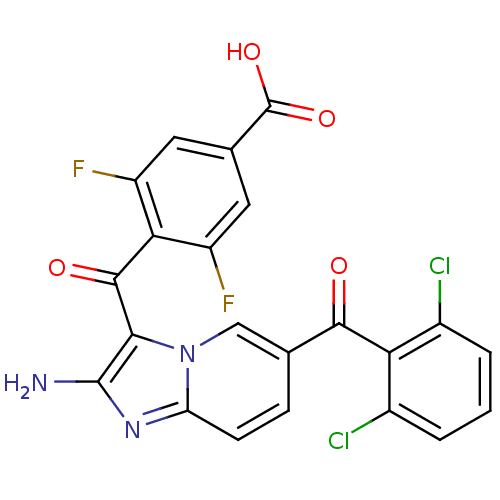 (4-({2-amino-6-[(2,6-dichlorophenyl)carbonyl]imidaz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6690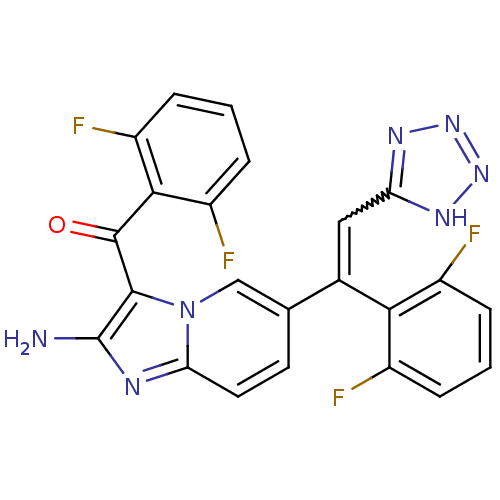 (6-[(Z)-1-(2,6-difluorophenyl)-2-(1H-1,2,3,4-tetraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM6678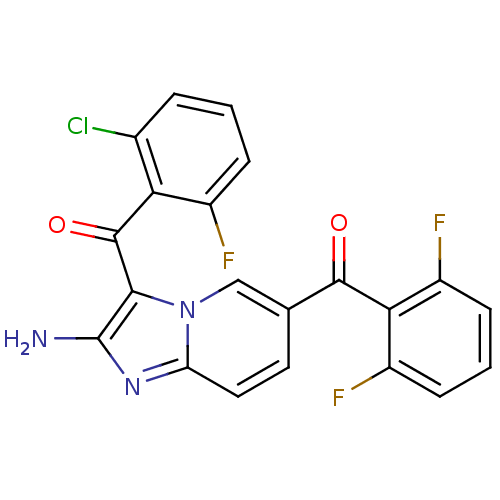 (3-[(2-chloro-6-fluorophenyl)carbonyl]-6-[(2,6-difl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6687 (6-[(2-chloro-6-fluorophenyl)carbonyl]-3-[(2,6-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | 25 |
Avenida de la Industria | Assay Description In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... | Bioorg Med Chem Lett 14: 6095-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.053 BindingDB Entry DOI: 10.7270/Q2ZP44BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 211 total ) | Next | Last >> |