 Found 391 hits with Last Name = 'tjandra' and Initial = 'm'
Found 391 hits with Last Name = 'tjandra' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile salt export pump
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030555
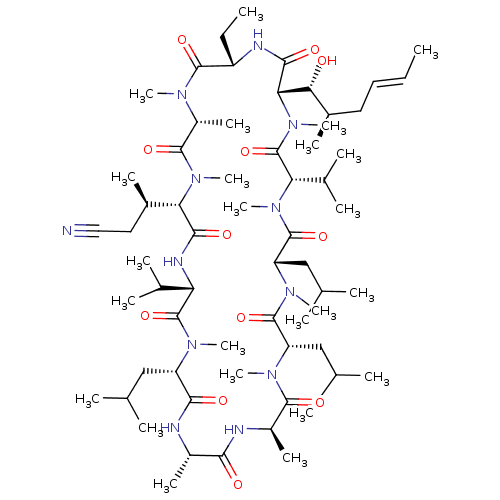
(CHEMBL3344493 | US9566312, Compound 2.10)Show SMILES [H][C@@]1([C@H](C)CC#N)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H110N12O12/c1-25-27-28-40(14)52(76)51-56(80)67-44(26-2)59(83)69(18)43(17)58(82)74(23)50(39(13)29-30-64)55(79)68-48(37(9)10)62(86)70(19)45(31-34(3)4)54(78)65-41(15)53(77)66-42(16)57(81)71(20)46(32-35(5)6)60(84)72(21)47(33-36(7)8)61(85)73(22)49(38(11)12)63(87)75(51)24/h25,27,34-52,76H,26,28-29,31-33H2,1-24H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b27-25+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030538
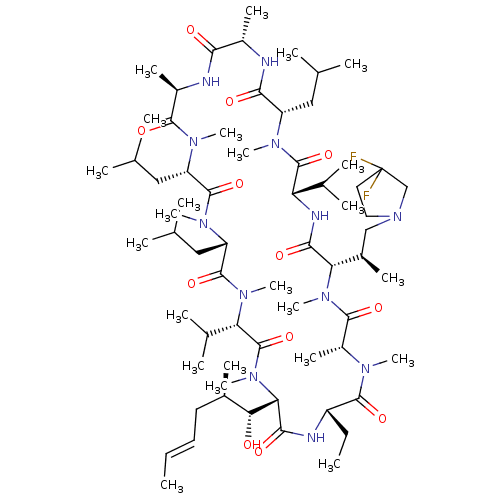
(CHEMBL3344497 | US9566312, Compound 2.5.18)Show SMILES [H][C@@]1([C@H](C)CN2CCC(F)(F)C2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C66H116F2N12O12/c1-25-27-28-41(13)54(81)53-58(85)71-46(26-2)61(88)73(18)45(17)60(87)78(23)52(42(14)34-80-30-29-66(67,68)35-80)57(84)72-50(39(9)10)64(91)74(19)47(31-36(3)4)56(83)69-43(15)55(82)70-44(16)59(86)75(20)48(32-37(5)6)62(89)76(21)49(33-38(7)8)63(90)77(22)51(40(11)12)65(92)79(53)24/h25,27,36-54,81H,26,28-35H2,1-24H3,(H,69,83)(H,70,82)(H,71,85)(H,72,84)/b27-25+/t41-,42-,43+,44-,45-,46+,47+,48+,49+,50+,51+,52+,53+,54-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030541
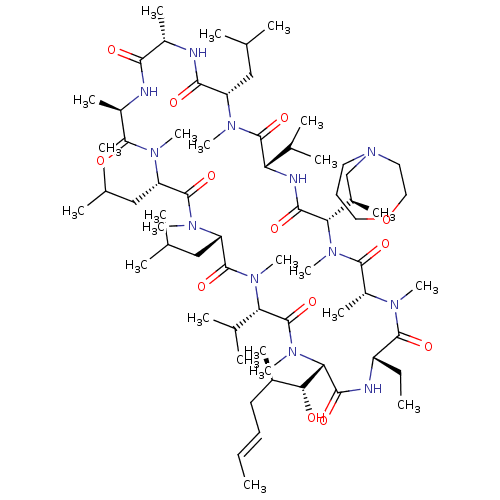
(CHEMBL3344496 | US9566312, Compound 2.5.21)Show SMILES [H][C@@]1([C@H](C)CN2CCCOCC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C67H120N12O13/c1-25-27-29-43(13)56(80)55-60(84)70-48(26-2)63(87)72(18)47(17)62(86)77(23)54(44(14)37-79-30-28-32-92-33-31-79)59(83)71-52(41(9)10)66(90)73(19)49(34-38(3)4)58(82)68-45(15)57(81)69-46(16)61(85)74(20)50(35-39(5)6)64(88)75(21)51(36-40(7)8)65(89)76(22)53(42(11)12)67(91)78(55)24/h25,27,38-56,80H,26,28-37H2,1-24H3,(H,68,82)(H,69,81)(H,70,84)(H,71,83)/b27-25+/t43-,44-,45+,46-,47-,48+,49+,50+,51+,52+,53+,54+,55+,56-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030536
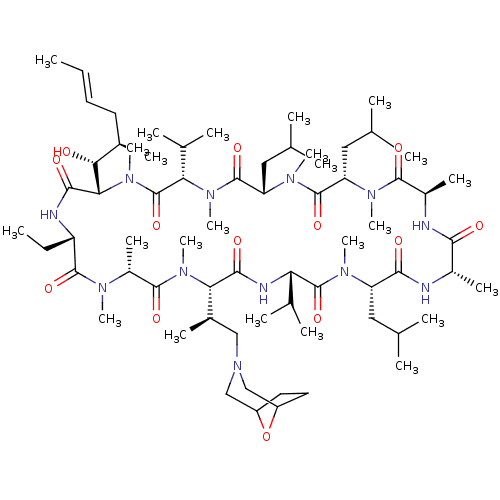
(CHEMBL3344498 | US9566312, Compound 2.5.3)Show SMILES [H][C@@]1([C@H](C)CN2CC3CCC(C2)O3)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C68H120N12O13/c1-25-27-28-42(13)57(81)56-61(85)71-49(26-2)64(88)73(18)46(17)63(87)78(23)55(43(14)34-80-35-47-29-30-48(36-80)93-47)60(84)72-53(40(9)10)67(91)74(19)50(31-37(3)4)59(83)69-44(15)58(82)70-45(16)62(86)75(20)51(32-38(5)6)65(89)76(21)52(33-39(7)8)66(90)77(22)54(41(11)12)68(92)79(56)24/h25,27,37-57,81H,26,28-36H2,1-24H3,(H,69,83)(H,70,82)(H,71,85)(H,72,84)/b27-25+/t42-,43-,44+,45-,46-,47?,48?,49+,50+,51+,52+,53+,54+,55+,56+,57-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030535
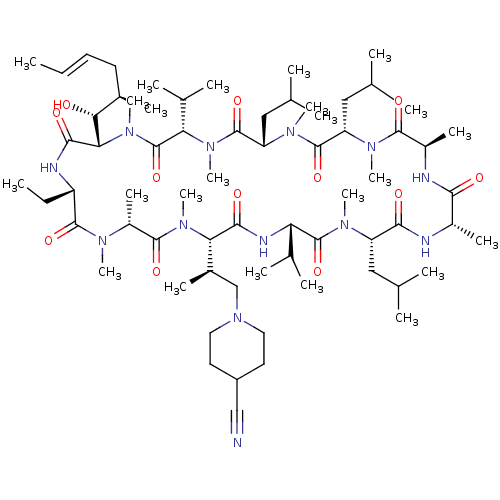
(CHEMBL3344499 | US9566312, Compound 2.5.26)Show SMILES [H][C@@]1([C@H](C)CN2CCC(CC2)C#N)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C68H119N13O12/c1-25-27-28-43(13)57(82)56-61(86)72-49(26-2)64(89)74(18)47(17)63(88)79(23)55(44(14)37-81-31-29-48(36-69)30-32-81)60(85)73-53(41(9)10)67(92)75(19)50(33-38(3)4)59(84)70-45(15)58(83)71-46(16)62(87)76(20)51(34-39(5)6)65(90)77(21)52(35-40(7)8)66(91)78(22)54(42(11)12)68(93)80(56)24/h25,27,38-57,82H,26,28-35,37H2,1-24H3,(H,70,84)(H,71,83)(H,72,86)(H,73,85)/b27-25+/t43-,44-,45+,46-,47-,49+,50+,51+,52+,53+,54+,55+,56+,57-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030534
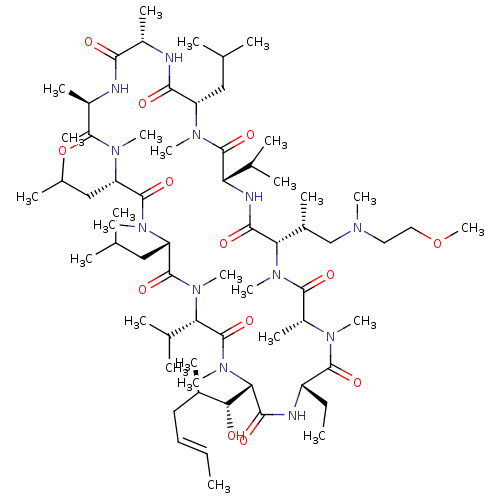
(CHEMBL3344500 | US9566312, Compound 2.5.22)Show SMILES [H][C@@]1([C@H](C)CN(C)CCOC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13/c1-27-29-30-42(13)55(79)54-59(83)69-47(28-2)62(86)72(19)46(17)61(85)77(24)53(43(14)36-71(18)31-32-91-26)58(82)70-51(40(9)10)65(89)73(20)48(33-37(3)4)57(81)67-44(15)56(80)68-45(16)60(84)74(21)49(34-38(5)6)63(87)75(22)50(35-39(7)8)64(88)76(23)52(41(11)12)66(90)78(54)25/h27,29,37-55,79H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43-,44+,45-,46-,47+,48+,49+,50+,51+,52+,53+,54+,55-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030554
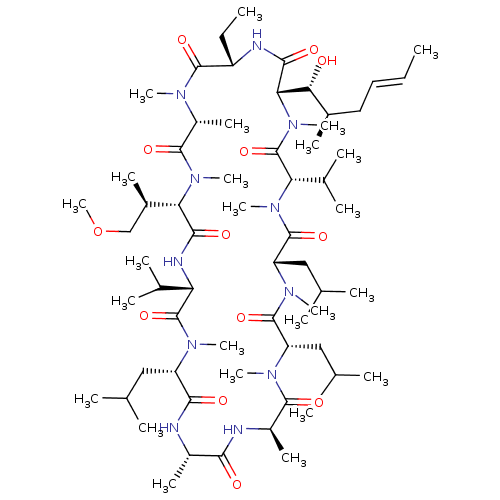
(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030533
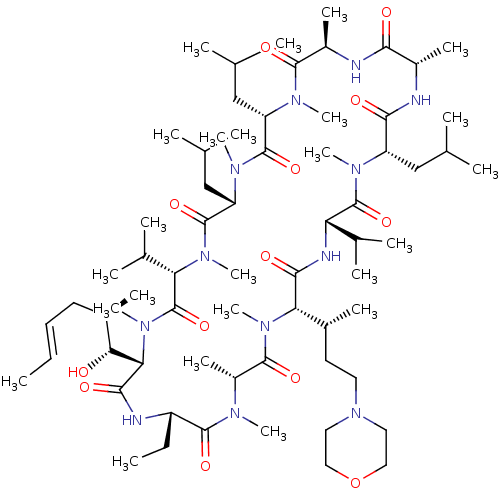
(CHEMBL3344502)Show SMILES [H][C@@]1([C@H](C)CCN2CCOCC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C67H120N12O13/c1-25-27-28-44(14)56(80)55-60(84)70-48(26-2)63(87)72(18)47(17)62(86)77(23)54(43(13)29-30-79-31-33-92-34-32-79)59(83)71-52(41(9)10)66(90)73(19)49(35-38(3)4)58(82)68-45(15)57(81)69-46(16)61(85)74(20)50(36-39(5)6)64(88)75(21)51(37-40(7)8)65(89)76(22)53(42(11)12)67(91)78(55)24/h25,27,38-56,80H,26,28-37H2,1-24H3,(H,68,82)(H,69,81)(H,70,84)(H,71,83)/b27-25+/t43-,44-,45+,46-,47-,48+,49+,50+,51+,52+,53+,54+,55+,56-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030542
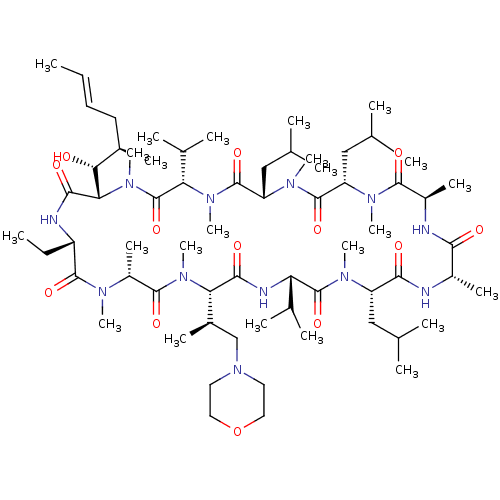
(CHEMBL3344495 | US9566312, Compound 2.5.2)Show SMILES [H][C@@]1([C@H](C)CN2CCOCC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C66H118N12O13/c1-25-27-28-42(13)55(79)54-59(83)69-47(26-2)62(86)71(18)46(17)61(85)76(23)53(43(14)36-78-29-31-91-32-30-78)58(82)70-51(40(9)10)65(89)72(19)48(33-37(3)4)57(81)67-44(15)56(80)68-45(16)60(84)73(20)49(34-38(5)6)63(87)74(21)50(35-39(7)8)64(88)75(22)52(41(11)12)66(90)77(54)24/h25,27,37-55,79H,26,28-36H2,1-24H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b27-25+/t42-,43-,44+,45-,46-,47+,48+,49+,50+,51+,52+,53+,54+,55-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50030494
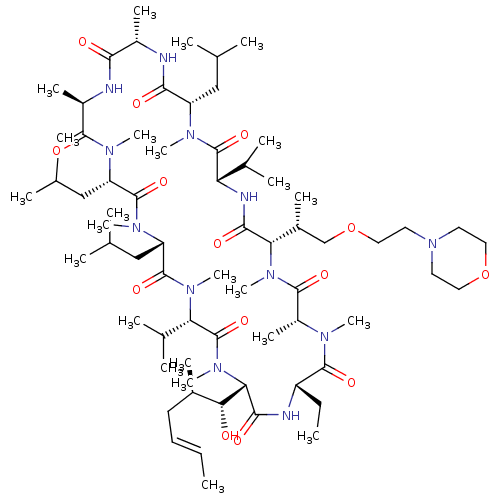
(CHEMBL3344503)Show SMILES [H][C@@]1([C@H](C)COCCN2CCOCC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C68H122N12O14/c1-25-27-28-44(13)57(81)56-61(85)71-49(26-2)64(88)73(18)48(17)63(87)78(23)55(45(14)38-94-34-31-80-29-32-93-33-30-80)60(84)72-53(42(9)10)67(91)74(19)50(35-39(3)4)59(83)69-46(15)58(82)70-47(16)62(86)75(20)51(36-40(5)6)65(89)76(21)52(37-41(7)8)66(90)77(22)54(43(11)12)68(92)79(56)24/h25,27,39-57,81H,26,28-38H2,1-24H3,(H,69,83)(H,70,82)(H,71,85)(H,72,84)/b27-25+/t44-,45-,46+,47-,48-,49+,50+,51+,52+,53+,54+,55+,56+,57-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2CJ (unknown origin) |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrate |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50454541

(CHEMBL4210493)Show SMILES NC(=N)NCc1cnn(C[C@@H]2[C@H](NC(=O)C(=N/OC3(CC3)C(O)=O)\c3csc(N)n3)C(=O)N2S(O)(=O)=O)n1 |r| Show InChI InChI=1S/C17H21N11O8S2/c18-15(19)21-3-7-4-22-27(25-7)5-9-11(13(30)28(9)38(33,34)35)24-12(29)10(8-6-37-16(20)23-8)26-36-17(1-2-17)14(31)32/h4,6,9,11H,1-3,5H2,(H2,20,23)(H,24,29)(H,31,32)(H4,18,19,21)(H,33,34,35)/b26-10-/t9-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA... |
Bioorg Med Chem Lett 28: 748-755 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.006
BindingDB Entry DOI: 10.7270/Q2M32ZB2 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50454543
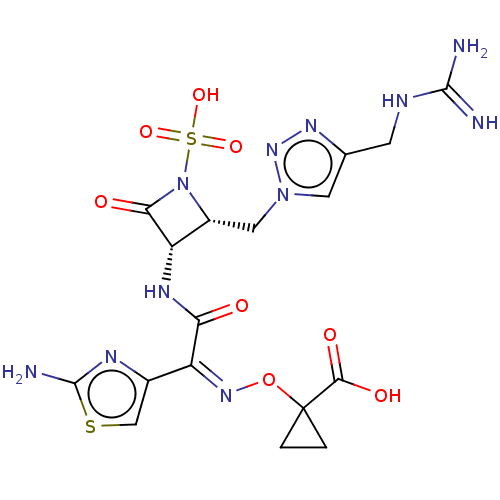
(CHEMBL4217850)Show SMILES NC(=N)NCc1cn(C[C@@H]2[C@H](NC(=O)C(=N/OC3(CC3)C(O)=O)\c3csc(N)n3)C(=O)N2S(O)(=O)=O)nn1 |r| Show InChI InChI=1S/C17H21N11O8S2/c18-15(19)21-3-7-4-27(26-24-7)5-9-11(13(30)28(9)38(33,34)35)23-12(29)10(8-6-37-16(20)22-8)25-36-17(1-2-17)14(31)32/h4,6,9,11H,1-3,5H2,(H2,20,22)(H,23,29)(H,31,32)(H4,18,19,21)(H,33,34,35)/b25-10-/t9-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA... |
Bioorg Med Chem Lett 28: 748-755 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.006
BindingDB Entry DOI: 10.7270/Q2M32ZB2 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50454540

(CHEMBL4213450)Show SMILES NC[C@@H]1CN(C[C@@H]2[C@H](NC(=O)C(=N/OC3(CC3)C(O)=O)\c3csc(N)n3)C(=O)N2S(O)(=O)=O)C(=O)O1 |r| Show InChI InChI=1S/C17H21N7O10S2/c18-3-7-4-23(16(29)33-7)5-9-11(13(26)24(9)36(30,31)32)21-12(25)10(8-6-35-15(19)20-8)22-34-17(1-2-17)14(27)28/h6-7,9,11H,1-5,18H2,(H2,19,20)(H,21,25)(H,27,28)(H,30,31,32)/b22-10-/t7-,9-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA... |
Bioorg Med Chem Lett 28: 748-755 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.006
BindingDB Entry DOI: 10.7270/Q2M32ZB2 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50454542

(CHEMBL4206129)Show SMILES Nc1nc(cs1)C(=N\OC1(CC1)C(O)=O)\C(=O)N[C@H]1[C@@H](CN2CCOC2=O)N(C1=O)S(O)(=O)=O |r| Show InChI InChI=1S/C16H18N6O10S2/c17-14-18-7(6-33-14)9(20-32-16(1-2-16)13(25)26)11(23)19-10-8(5-21-3-4-31-15(21)27)22(12(10)24)34(28,29)30/h6,8,10H,1-5H2,(H2,17,18)(H,19,23)(H,25,26)(H,28,29,30)/b20-9-/t8-,10+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA... |
Bioorg Med Chem Lett 28: 748-755 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.006
BindingDB Entry DOI: 10.7270/Q2M32ZB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50030554

(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 2.05 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50030554

(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 1.45 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM50030554

(CHEMBL3344494 | US9566312, Compound 2.1)Show SMILES [H][C@@]1([C@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286505

(US9566312, Compound 2.2)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40+,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286505

(US9566312, Compound 2.2)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40+,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286505

(US9566312, Compound 2.2)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@@H](C)COC)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O13/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-87-25)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40+,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286506

(US9566312, Compound 2.3)Show SMILES CCOC[C@@H](C)[C@@H]1N2CN([C@@H](C(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@H](O)[C@H](C)C\C=C\C)C(=O)N[C@@H](CC)C(=O)N(C)[C@H](C)C2=O)C1=O |r| Show InChI InChI=1S/C64H113N11O13/c1-25-28-29-40(14)53(76)52-56(79)67-45(26-2)59(82)68(19)44(18)58(81)75-34-74(64(87)51(75)41(15)33-88-27-3)50(39(12)13)63(86)69(20)46(30-35(4)5)55(78)65-42(16)54(77)66-43(17)57(80)70(21)47(31-36(6)7)60(83)71(22)48(32-37(8)9)61(84)72(23)49(38(10)11)62(85)73(52)24/h25,28,35-53,76H,26-27,29-34H2,1-24H3,(H,65,78)(H,66,77)(H,67,79)/b28-25+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 12.7 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286506

(US9566312, Compound 2.3)Show SMILES CCOC[C@@H](C)[C@@H]1N2CN([C@@H](C(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@H](O)[C@H](C)C\C=C\C)C(=O)N[C@@H](CC)C(=O)N(C)[C@H](C)C2=O)C1=O |r| Show InChI InChI=1S/C64H113N11O13/c1-25-28-29-40(14)53(76)52-56(79)67-45(26-2)59(82)68(19)44(18)58(81)75-34-74(64(87)51(75)41(15)33-88-27-3)50(39(12)13)63(86)69(20)46(30-35(4)5)55(78)65-42(16)54(77)66-43(17)57(80)70(21)47(31-36(6)7)60(83)71(22)48(32-37(8)9)61(84)72(23)49(38(10)11)62(85)73(52)24/h25,28,35-53,76H,26-27,29-34H2,1-24H3,(H,65,78)(H,66,77)(H,67,79)/b28-25+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286506

(US9566312, Compound 2.3)Show SMILES CCOC[C@@H](C)[C@@H]1N2CN([C@@H](C(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](CC(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@H](O)[C@H](C)C\C=C\C)C(=O)N[C@@H](CC)C(=O)N(C)[C@H](C)C2=O)C1=O |r| Show InChI InChI=1S/C64H113N11O13/c1-25-28-29-40(14)53(76)52-56(79)67-45(26-2)59(82)68(19)44(18)58(81)75-34-74(64(87)51(75)41(15)33-88-27-3)50(39(12)13)63(86)69(20)46(30-35(4)5)55(78)65-42(16)54(77)66-43(17)57(80)70(21)47(31-36(6)7)60(83)71(22)48(32-37(8)9)61(84)72(23)49(38(10)11)62(85)73(52)24/h25,28,35-53,76H,26-27,29-34H2,1-24H3,(H,65,78)(H,66,77)(H,67,79)/b28-25+/t40-,41-,42+,43-,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM286507
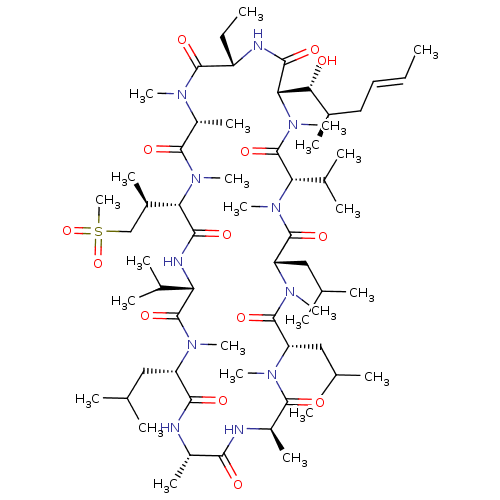
(US9566312, Compound 2.4)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CS(C)(=O)=O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14S/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-89(25,87)88)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM286507

(US9566312, Compound 2.4)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CS(C)(=O)=O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14S/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-89(25,87)88)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase F, mitochondrial
(Homo sapiens (Human)) | BDBM286507

(US9566312, Compound 2.4)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H]([C@H](C)CS(C)(=O)=O)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O14S/c1-26-28-29-39(13)52(75)51-56(79)66-44(27-2)59(82)68(18)43(17)58(81)73(23)50(40(14)33-89(25,87)88)55(78)67-48(37(9)10)62(85)69(19)45(30-34(3)4)54(77)64-41(15)53(76)65-42(16)57(80)70(20)46(31-35(5)6)60(83)71(21)47(32-36(7)8)61(84)72(22)49(38(11)12)63(86)74(51)24/h26,28,34-52,75H,27,29-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t39-,40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | 7.4 | n/a |
Novartis AG
US Patent
| Assay Description
Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro... |
US Patent US9566312 (2017)
BindingDB Entry DOI: 10.7270/Q2K939J1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by surface plasmon resonance method |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50339126
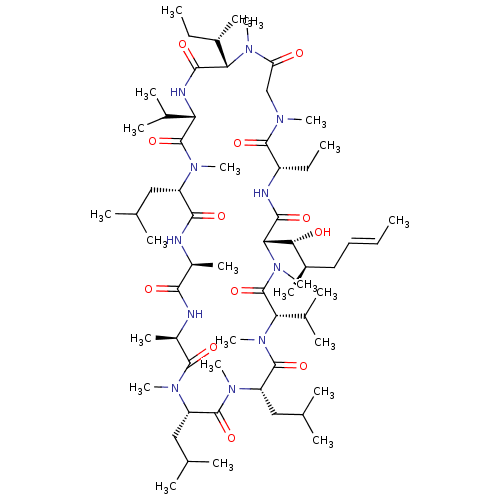
((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-24-sec-buty...)Show SMILES CC[C@H](C)[C@@H]1N(C)C(=O)CN(C)C(=O)[C@H](CC)NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-28-29-40(15)52(75)51-56(79)65-43(27-3)58(81)67(18)33-47(74)71(22)50(39(14)26-2)55(78)66-48(37(10)11)61(84)68(19)44(30-34(4)5)54(77)63-41(16)53(76)64-42(17)57(80)69(20)45(31-35(6)7)59(82)70(21)46(32-36(8)9)60(83)72(23)49(38(12)13)62(85)73(51)24/h25,28,34-46,48-52,75H,26-27,29-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b28-25+/t39-,40+,41-,42+,43-,44-,45-,46-,48-,49-,50-,51-,52+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by surface plasmon resonance method |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50339126

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-24-sec-buty...)Show SMILES CC[C@H](C)[C@@H]1N(C)C(=O)CN(C)C(=O)[C@H](CC)NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-28-29-40(15)52(75)51-56(79)65-43(27-3)58(81)67(18)33-47(74)71(22)50(39(14)26-2)55(78)66-48(37(10)11)61(84)68(19)44(30-34(4)5)54(77)63-41(16)53(76)64-42(17)57(80)69(20)45(31-35(6)7)59(82)70(21)46(32-36(8)9)60(83)72(23)49(38(12)13)62(85)73(51)24/h25,28,34-46,48-52,75H,26-27,29-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b28-25+/t39-,40+,41-,42+,43-,44-,45-,46-,48-,49-,50-,51-,52+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin B by surface plasmon resonance method |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin B by surface plasmon resonance method |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50323721
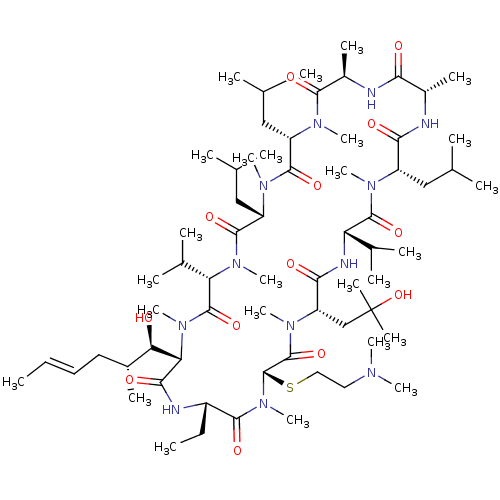
((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin B by surface plasmon resonance method |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM50030493

(CHEMBL3344501 | US9566312, Compound 2.17.4)Show SMILES [H][C@@]1([C@@H](C)CN2CCN(CCOC)CC2)N(C)C(=O)[C@@H](C)N(C)C(=O)[C@H](CC)NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC1=O)C(C)C |r| Show InChI InChI=1S/C69H125N13O13/c1-26-28-29-45(13)58(83)57-62(87)72-50(27-2)65(90)74(18)49(17)64(89)79(23)56(46(14)39-82-32-30-81(31-33-82)34-35-95-25)61(86)73-54(43(9)10)68(93)75(19)51(36-40(3)4)60(85)70-47(15)59(84)71-48(16)63(88)76(20)52(37-41(5)6)66(91)77(21)53(38-42(7)8)67(92)78(22)55(44(11)12)69(94)80(57)24/h26,28,40-58,83H,27,29-39H2,1-25H3,(H,70,85)(H,71,84)(H,72,87)(H,73,86)/b28-26+/t45-,46+,47+,48-,49-,50+,51+,52+,53+,54+,55+,56+,57+,58-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin B by surface plasmon resonance method |
J Med Chem 57: 8503-16 (2014)
Article DOI: 10.1021/jm500862r
BindingDB Entry DOI: 10.7270/Q2HM5B1Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































