

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513317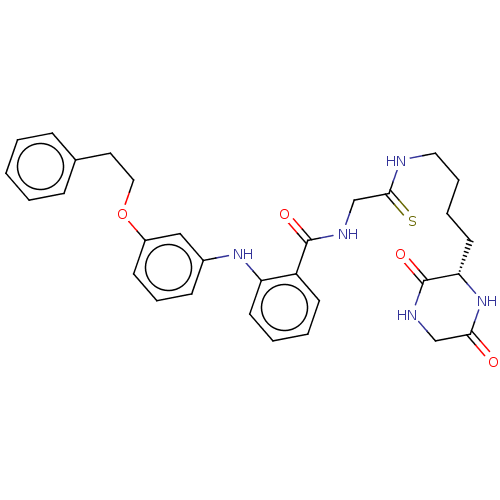 (CHEMBL4465620) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513318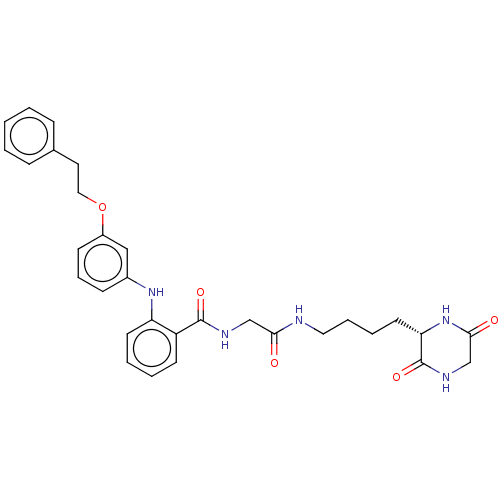 (CHEMBL4516553) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50435711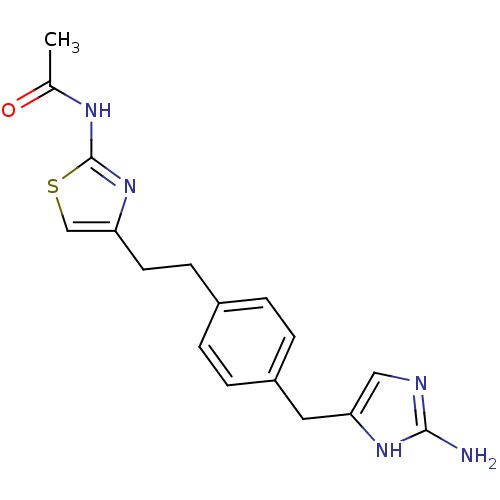 (CHEMBL2392121) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50427008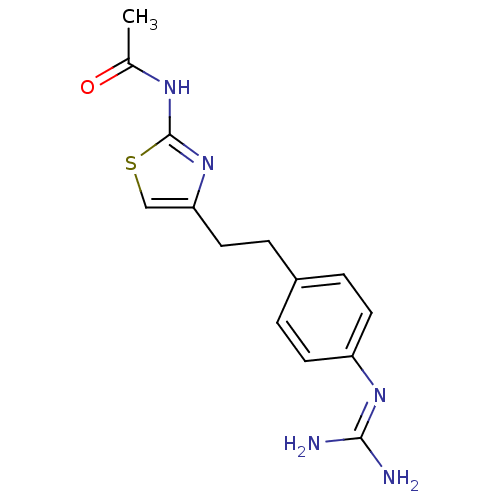 (CHEMBL2326864) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50427008 (CHEMBL2326864) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50435712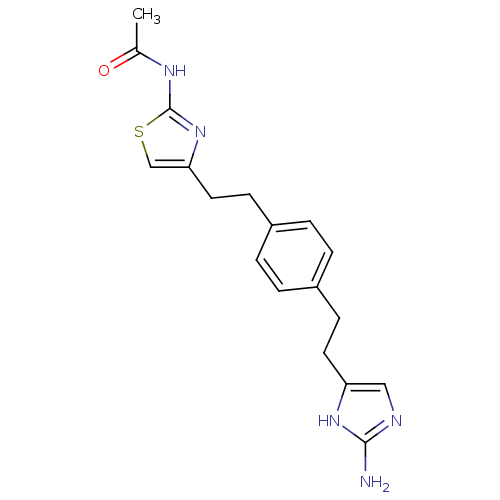 (CHEMBL2390968) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433263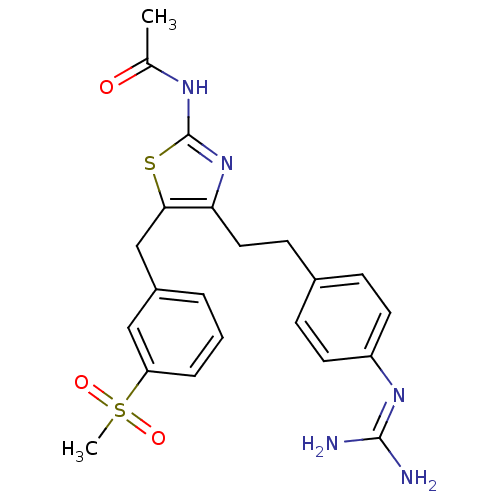 (CHEMBL2376162) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50435711 (CHEMBL2392121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured a... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433257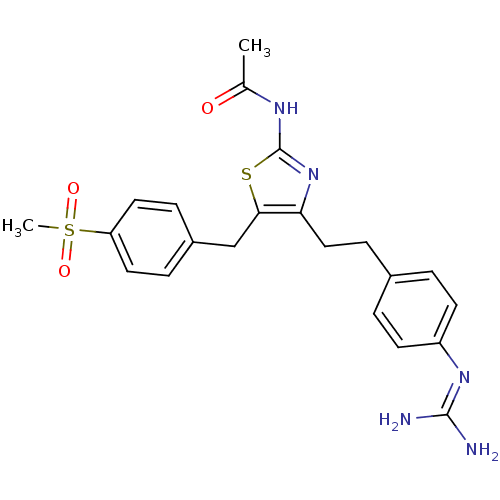 (CHEMBL2376160 | CHEMBL2376161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433266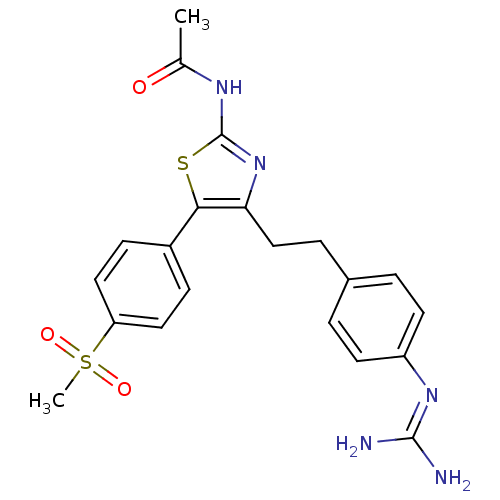 (CHEMBL2376169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433263 (CHEMBL2376162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433258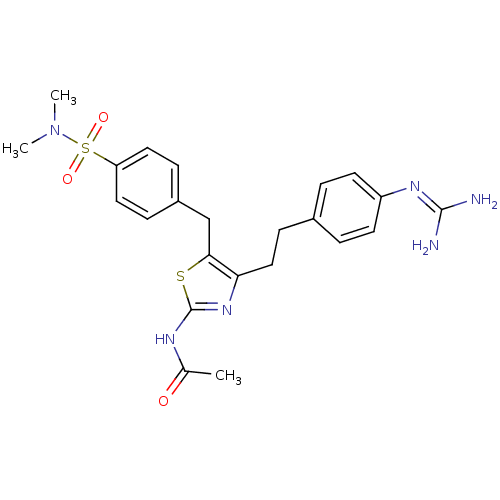 (CHEMBL2376166) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433259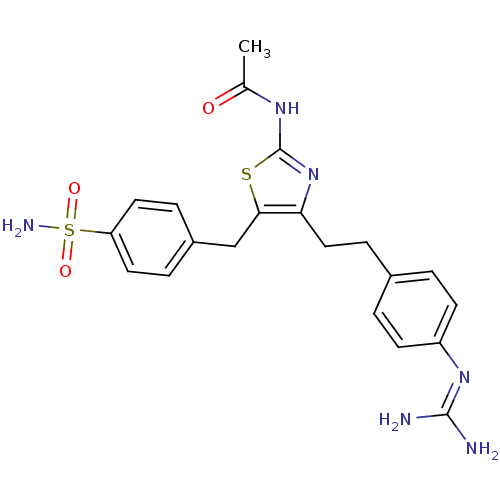 (CHEMBL2375367) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433257 (CHEMBL2376160 | CHEMBL2376161) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433261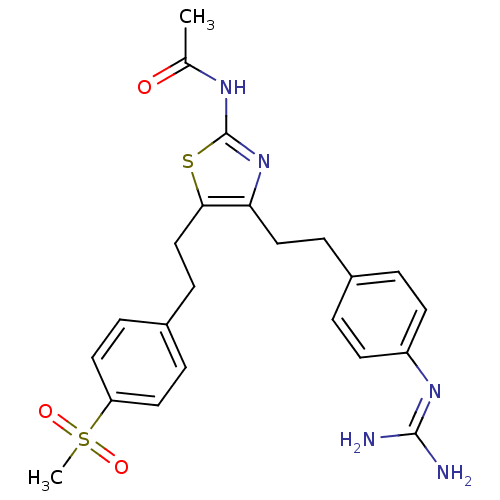 (CHEMBL2376164) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433259 (CHEMBL2375367) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50435707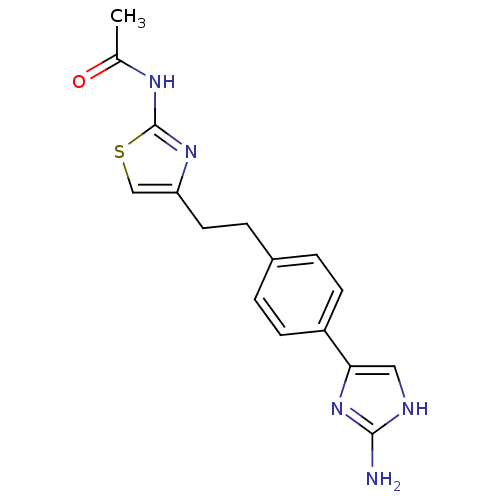 (CHEMBL2392120) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433258 (CHEMBL2376166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433261 (CHEMBL2376164) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433262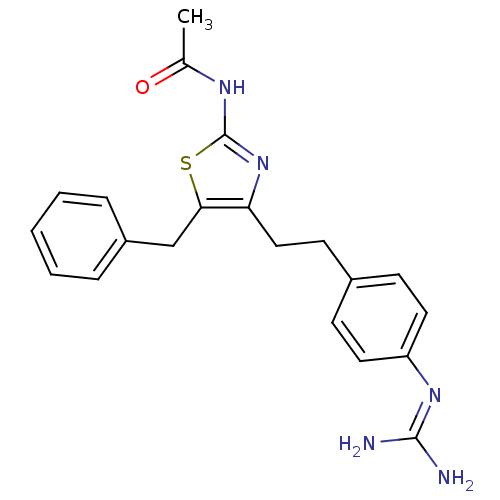 (CHEMBL2376163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427008 (CHEMBL2326864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured a... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427008 (CHEMBL2326864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50151585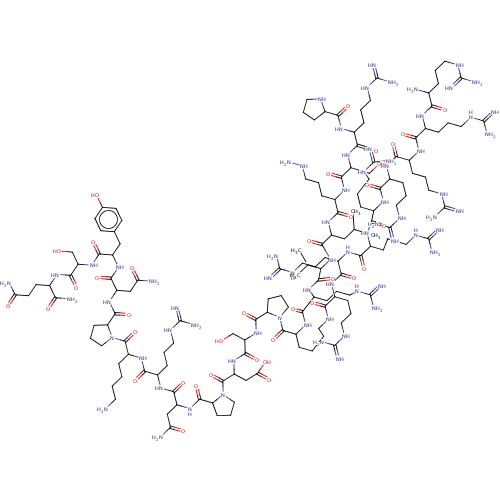 (CHEMBL3774476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged human recombinant LSD1 (1 to 852 residues) expressed in Escherichia coli BL21(DE3) cells using H3K4me... | J Med Chem 59: 1531-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01323 BindingDB Entry DOI: 10.7270/Q2JH3P22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50151584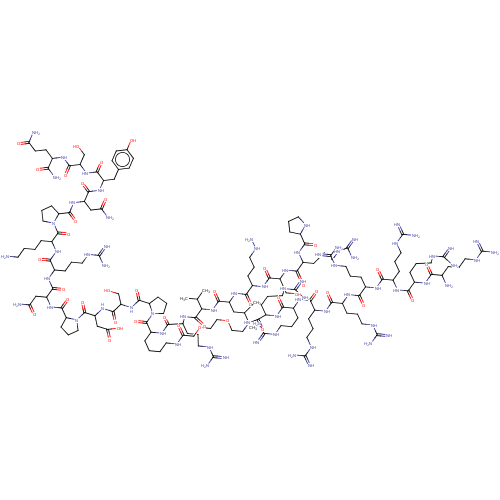 (CHEMBL3775613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged human recombinant LSD1 (1 to 852 residues) expressed in Escherichia coli BL21(DE3) cells using H3K4me... | J Med Chem 59: 1531-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01323 BindingDB Entry DOI: 10.7270/Q2JH3P22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433262 (CHEMBL2376163) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50148781 (CHEMBL3770903) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513317 (CHEMBL4465620) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50151583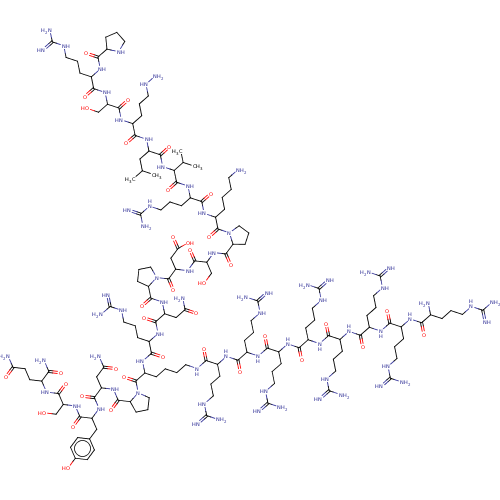 (CHEMBL3774920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged human recombinant LSD1 (1 to 852 residues) expressed in Escherichia coli BL21(DE3) cells using H3K4me... | J Med Chem 59: 1531-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01323 BindingDB Entry DOI: 10.7270/Q2JH3P22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433260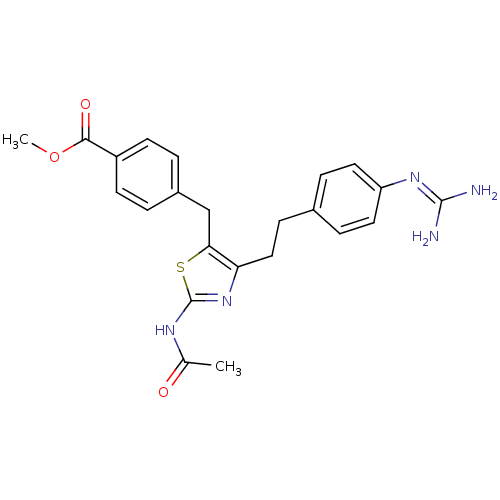 (CHEMBL2376165) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427009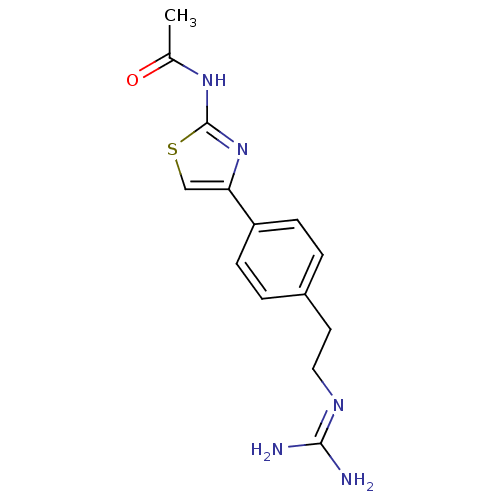 (CHEMBL2326875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513320 (CHEMBL4471909) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433267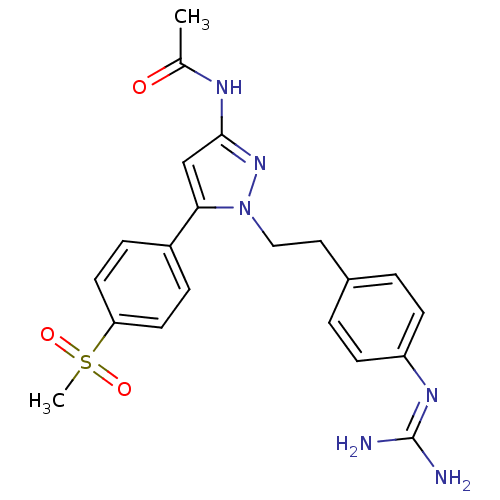 (CHEMBL2376168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50435706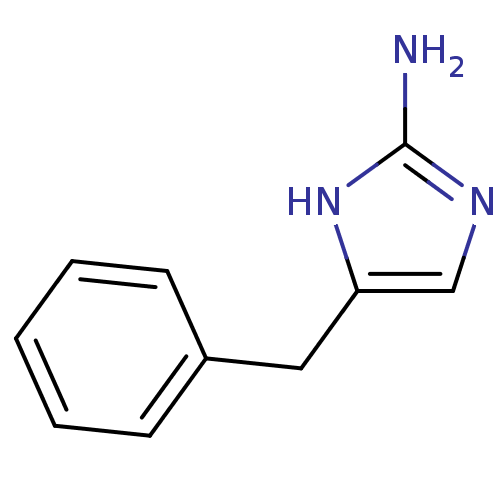 (CHEMBL2392118) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50151587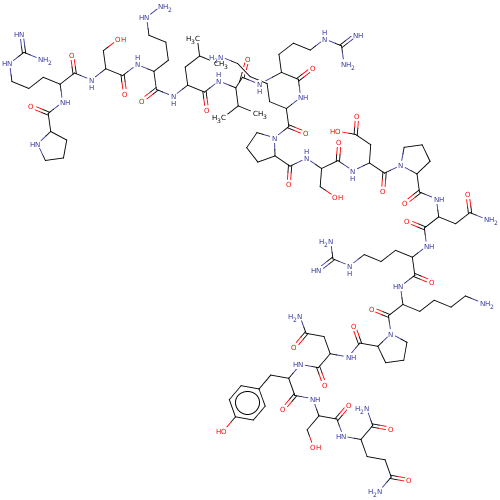 (CHEMBL3774486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged human recombinant LSD1 (1 to 852 residues) expressed in Escherichia coli BL21(DE3) cells using H3K4me... | J Med Chem 59: 1531-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01323 BindingDB Entry DOI: 10.7270/Q2JH3P22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433260 (CHEMBL2376165) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427014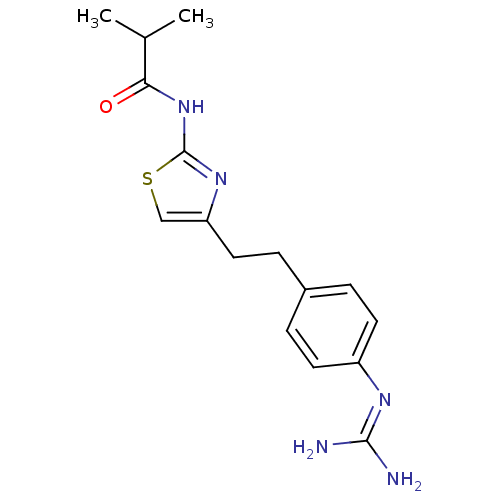 (CHEMBL2326870) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513318 (CHEMBL4516553) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50151588 (CHEMBL3775735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged human recombinant LSD1 (1 to 852 residues) expressed in Escherichia coli BL21(DE3) cells using H3K4me... | J Med Chem 59: 1531-44 (2016) Article DOI: 10.1021/acs.jmedchem.5b01323 BindingDB Entry DOI: 10.7270/Q2JH3P22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427022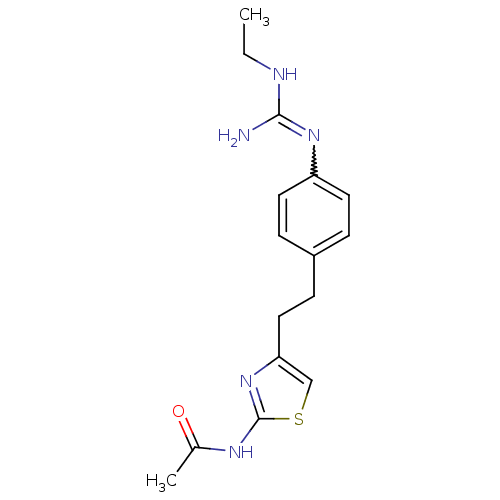 (CHEMBL2326861) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427019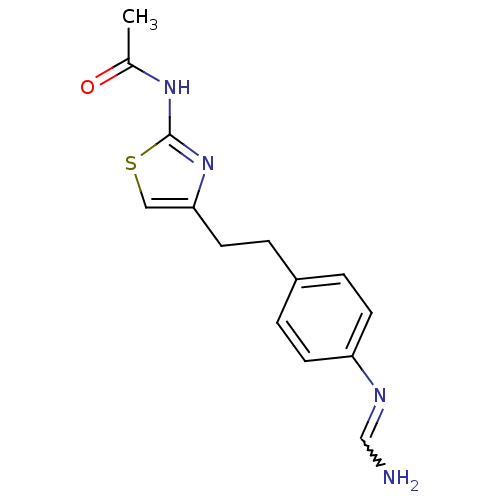 (CHEMBL2326865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50433265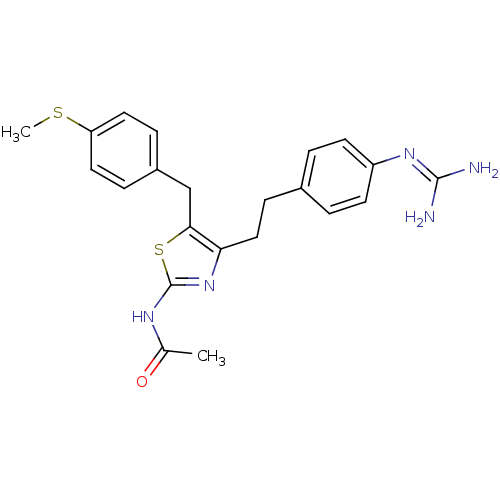 (CHEMBL2376170) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after 1... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM50435710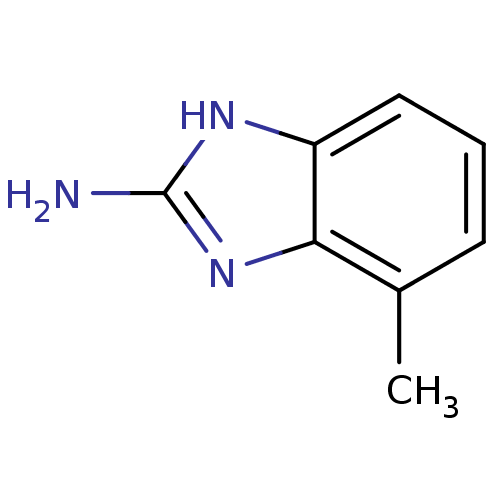 (CHEMBL2392117) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM7960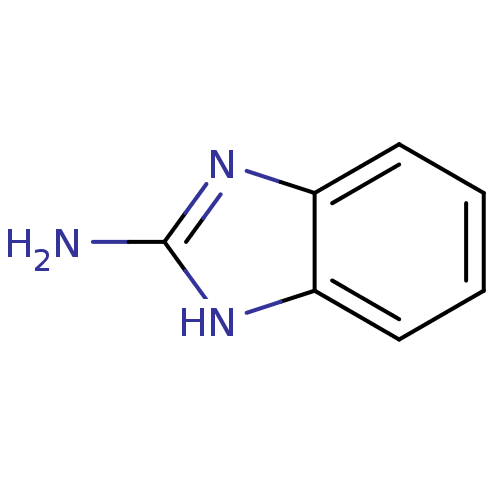 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433265 (CHEMBL2376170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427024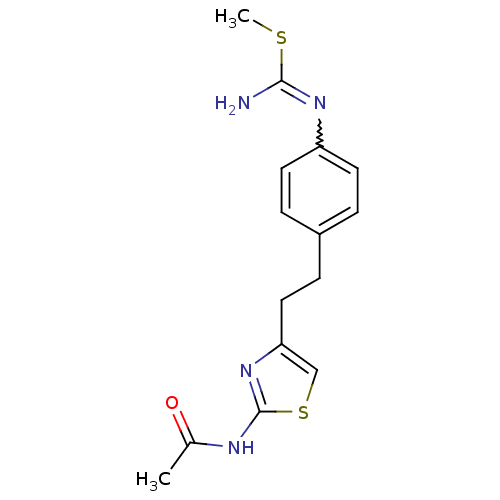 (CHEMBL2326859) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50427021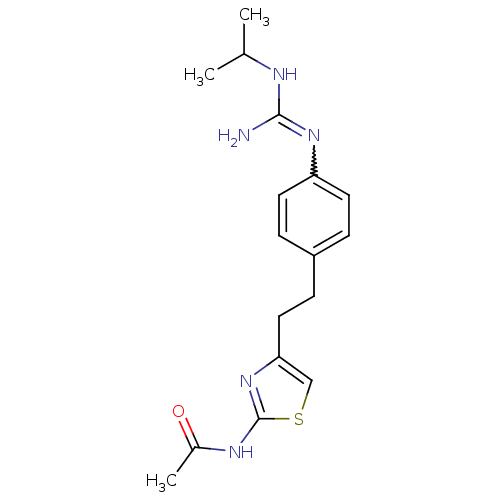 (CHEMBL2326862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 1219-33 (2013) Article DOI: 10.1016/j.bmc.2012.12.025 BindingDB Entry DOI: 10.7270/Q2K64KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513319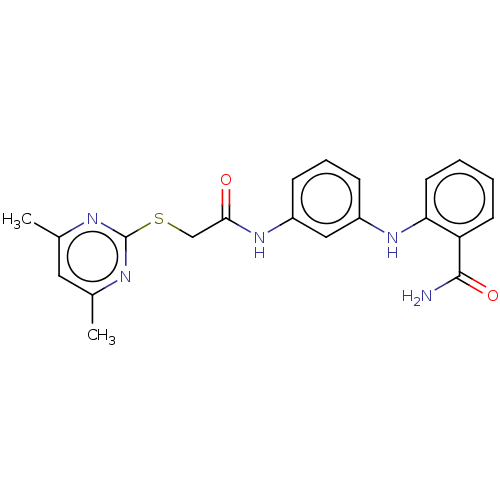 (CHEMBL4589851) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50392111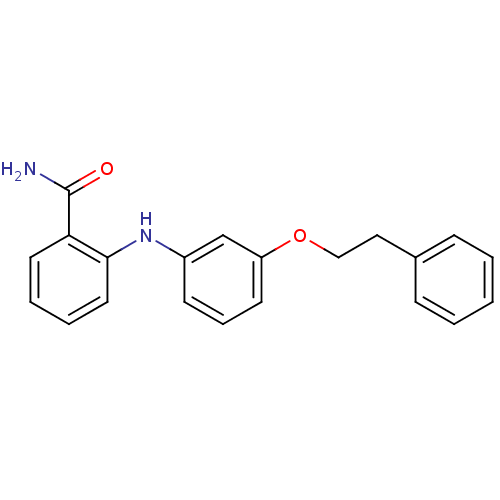 (CHEMBL2152613) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM50095454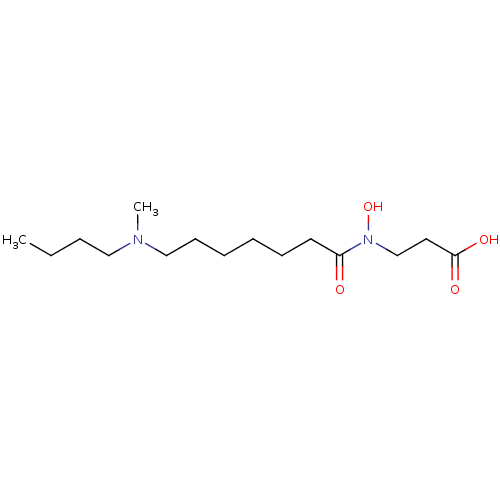 (CHEMBL3590422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of JARID1A (unknown origin) using biotinylated H3K9mel peptide after 60 mins by Alpha screen assay | ACS Med Chem Lett 6: 665-70 (2015) Article DOI: 10.1021/acsmedchemlett.5b00083 BindingDB Entry DOI: 10.7270/Q2V69MC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM50433268 (CHEMBL2376167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP1 expressed in CHO cells using [14C]-benzylamine as substrate incubated for 30 mins prior to substrate addition measured after... | Bioorg Med Chem 21: 2478-94 (2013) Article DOI: 10.1016/j.bmc.2013.02.048 BindingDB Entry DOI: 10.7270/Q2M046TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 175 total ) | Next | Last >> |