 Found 29 hits with Last Name = 'tyms' and Initial = 'as'
Found 29 hits with Last Name = 'tyms' and Initial = 'as' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283355
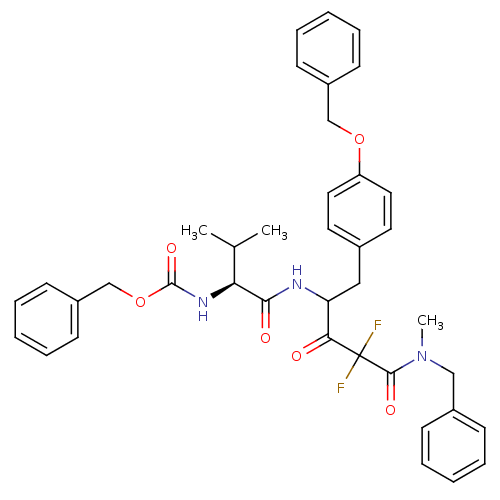
(CHEMBL311418 | {(S)-1-[3-(Benzyl-methyl-carbamoyl)...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C39H41F2N3O6/c1-27(2)34(43-38(48)50-26-31-17-11-6-12-18-31)36(46)42-33(23-28-19-21-32(22-20-28)49-25-30-15-9-5-10-16-30)35(45)39(40,41)37(47)44(3)24-29-13-7-4-8-14-29/h4-22,27,33-34H,23-26H2,1-3H3,(H,42,46)(H,43,48)/t33?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283358

(CHEMBL79799 | [(S)-1-(1-Benzyl-3-benzylcarbamoyl-3...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H33F2N3O5/c1-21(2)26(36-30(40)41-20-24-16-10-5-11-17-24)28(38)35-25(18-22-12-6-3-7-13-22)27(37)31(32,33)29(39)34-19-23-14-8-4-9-15-23/h3-17,21,25-26H,18-20H2,1-2H3,(H,34,39)(H,35,38)(H,36,40)/t25?,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037811

(CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C38H39F2N3O6/c1-26(2)33(43-37(47)49-25-30-16-10-5-11-17-30)35(45)42-32(34(44)38(39,40)36(46)41-23-28-12-6-3-7-13-28)22-27-18-20-31(21-19-27)48-24-29-14-8-4-9-15-29/h3-21,26,32-33H,22-25H2,1-2H3,(H,41,46)(H,42,45)(H,43,47)/t32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was determined against HIV-1 protease |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283362

(CHEMBL311049 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)N1CCOCC1 Show InChI InChI=1S/C35H39F2N3O7/c1-24(2)30(39-34(44)47-23-27-11-7-4-8-12-27)32(42)38-29(31(41)35(36,37)33(43)40-17-19-45-20-18-40)21-25-13-15-28(16-14-25)46-22-26-9-5-3-6-10-26/h3-16,24,29-30H,17-23H2,1-2H3,(H,38,42)(H,39,44)/t29?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037811

(CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C38H39F2N3O6/c1-26(2)33(43-37(47)49-25-30-16-10-5-11-17-30)35(45)42-32(34(44)38(39,40)36(46)41-23-28-12-6-3-7-13-28)22-27-18-20-31(21-19-27)48-24-29-14-8-4-9-15-29/h3-21,26,32-33H,22-25H2,1-2H3,(H,41,46)(H,42,45)(H,43,47)/t32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282534

(CHEMBL21095 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3-((R...)Show SMILES CC(C)[C@H](COCc1ccccc1)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H49F2N3O7/c1-29(2)37(28-53-25-32-14-8-5-9-15-32)47-41(51)43(44,45)39(49)36(24-31-20-22-35(23-21-31)54-26-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)55-27-34-18-12-7-13-19-34/h5-23,29-30,36-38H,24-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36?,37-,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was determined against HIV-1 protease |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283359
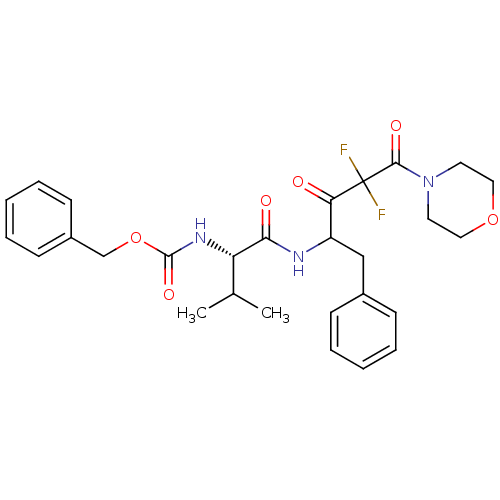
(CHEMBL83002 | [(S)-1-(1-Benzyl-3,3-difluoro-4-morp...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)N1CCOCC1 Show InChI InChI=1S/C28H33F2N3O6/c1-19(2)23(32-27(37)39-18-21-11-7-4-8-12-21)25(35)31-22(17-20-9-5-3-6-10-20)24(34)28(29,30)26(36)33-13-15-38-16-14-33/h3-12,19,22-23H,13-18H2,1-2H3,(H,31,35)(H,32,37)/t22?,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282525
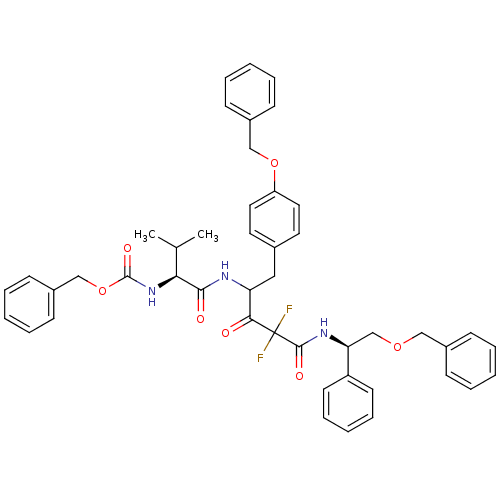
(CHEMBL283537 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3-((...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)N[C@@H](COCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C46H47F2N3O7/c1-32(2)41(51-45(55)58-30-36-19-11-5-12-20-36)43(53)49-39(27-33-23-25-38(26-24-33)57-29-35-17-9-4-10-18-35)42(52)46(47,48)44(54)50-40(37-21-13-6-14-22-37)31-56-28-34-15-7-3-8-16-34/h3-26,32,39-41H,27-31H2,1-2H3,(H,49,53)(H,50,54)(H,51,55)/t39?,40-,41-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282527
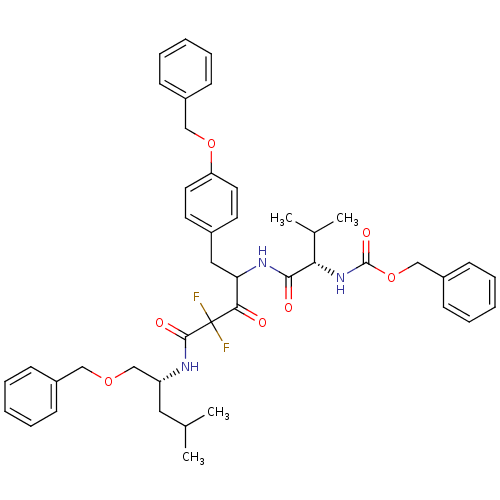
(CHEMBL280358 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3-((...)Show SMILES CC(C)C[C@H](COCc1ccccc1)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C44H51F2N3O7/c1-30(2)24-36(29-54-26-33-14-8-5-9-15-33)47-42(52)44(45,46)40(50)38(25-32-20-22-37(23-21-32)55-27-34-16-10-6-11-17-34)48-41(51)39(31(3)4)49-43(53)56-28-35-18-12-7-13-19-35/h5-23,30-31,36,38-39H,24-29H2,1-4H3,(H,47,52)(H,48,51)(H,49,53)/t36-,38?,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283364
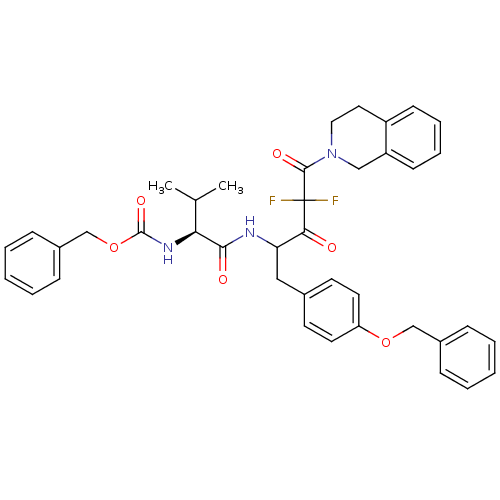
(CHEMBL314234 | {(S)-1-[1-(4-Benzyloxy-benzyl)-4-(3...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)N1CCc2ccccc2C1 Show InChI InChI=1S/C40H41F2N3O6/c1-27(2)35(44-39(49)51-26-30-13-7-4-8-14-30)37(47)43-34(23-28-17-19-33(20-18-28)50-25-29-11-5-3-6-12-29)36(46)40(41,42)38(48)45-22-21-31-15-9-10-16-32(31)24-45/h3-20,27,34-35H,21-26H2,1-2H3,(H,43,47)(H,44,49)/t34?,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282538
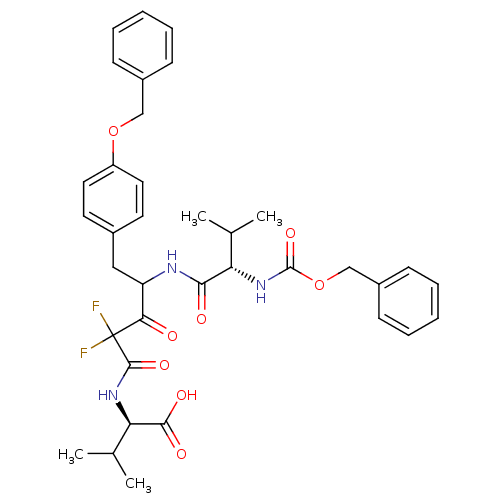
((R)-2-[4-((S)-2-Benzyloxycarbonylamino-3-methyl-bu...)Show SMILES CC(C)[C@@H](NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(O)=O Show InChI InChI=1S/C36H41F2N3O8/c1-22(2)29(41-35(47)49-21-26-13-9-6-10-14-26)32(43)39-28(31(42)36(37,38)34(46)40-30(23(3)4)33(44)45)19-24-15-17-27(18-16-24)48-20-25-11-7-5-8-12-25/h5-18,22-23,28-30H,19-21H2,1-4H3,(H,39,43)(H,40,46)(H,41,47)(H,44,45)/t28?,29-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282526
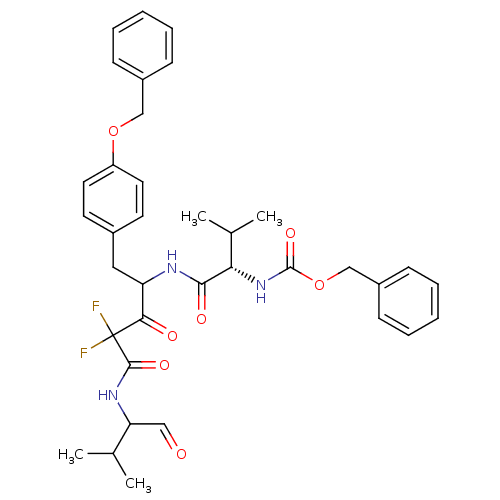
(CHEMBL20642 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-d...)Show SMILES CC(C)C(NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C=O Show InChI InChI=1S/C36H41F2N3O7/c1-23(2)30(20-42)40-34(45)36(37,38)32(43)29(19-25-15-17-28(18-16-25)47-21-26-11-7-5-8-12-26)39-33(44)31(24(3)4)41-35(46)48-22-27-13-9-6-10-14-27/h5-18,20,23-24,29-31H,19,21-22H2,1-4H3,(H,39,44)(H,40,45)(H,41,46)/t29?,30?,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282537
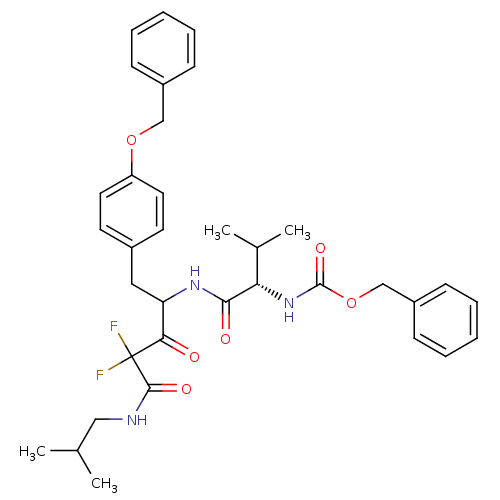
(CHEMBL264739 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-...)Show SMILES CC(C)CNC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C35H41F2N3O6/c1-23(2)20-38-33(43)35(36,37)31(41)29(19-25-15-17-28(18-16-25)45-21-26-11-7-5-8-12-26)39-32(42)30(24(3)4)40-34(44)46-22-27-13-9-6-10-14-27/h5-18,23-24,29-30H,19-22H2,1-4H3,(H,38,43)(H,39,42)(H,40,44)/t29?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282529

(CHEMBL19752 | {(S)-1-[(R)-1-(4-Benzyloxy-benzyl)-3...)Show SMILES CC(C)[C@H](COCc1ccccc1)NC(=O)C(F)(F)[C@H](O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H51F2N3O7/c1-29(2)37(28-53-25-32-14-8-5-9-15-32)47-41(51)43(44,45)39(49)36(24-31-20-22-35(23-21-31)54-26-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)55-27-34-18-12-7-13-19-34/h5-23,29-30,36-39,49H,24-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36?,37-,38-,39+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282535

(CHEMBL19973 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-d...)Show SMILES CC(C)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C34H39F2N3O6/c1-22(2)29(39-33(43)45-21-26-13-9-6-10-14-26)31(41)38-28(30(40)34(35,36)32(42)37-23(3)4)19-24-15-17-27(18-16-24)44-20-25-11-7-5-8-12-25/h5-18,22-23,28-29H,19-21H2,1-4H3,(H,37,42)(H,38,41)(H,39,43)/t28?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282528
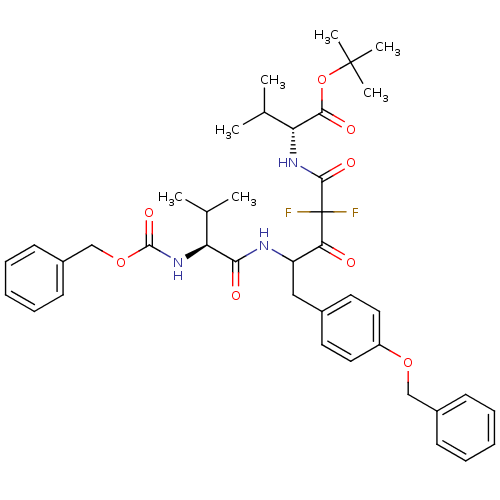
((R)-2-[4-((S)-2-Benzyloxycarbonylamino-3-methyl-bu...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)N[C@H](C(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C40H49F2N3O8/c1-25(2)32(45-38(50)52-24-29-16-12-9-13-17-29)35(47)43-31(22-27-18-20-30(21-19-27)51-23-28-14-10-8-11-15-28)34(46)40(41,42)37(49)44-33(26(3)4)36(48)53-39(5,6)7/h8-21,25-26,31-33H,22-24H2,1-7H3,(H,43,47)(H,44,49)(H,45,50)/t31?,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283357

(CHEMBL82829 | {[3-Benzylcarbamoyl-1-(4-benzyloxy-b...)Show SMILES FC(F)(C(=O)NCc1ccccc1)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)C(NC(=O)OCc1ccccc1)C1CCCC1 Show InChI InChI=1S/C40H41F2N3O6/c41-40(42,38(48)43-25-29-12-4-1-5-13-29)36(46)34(24-28-20-22-33(23-21-28)50-26-30-14-6-2-7-15-30)44-37(47)35(32-18-10-11-19-32)45-39(49)51-27-31-16-8-3-9-17-31/h1-9,12-17,20-23,32,34-35H,10-11,18-19,24-27H2,(H,43,48)(H,44,47)(H,45,49) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282531
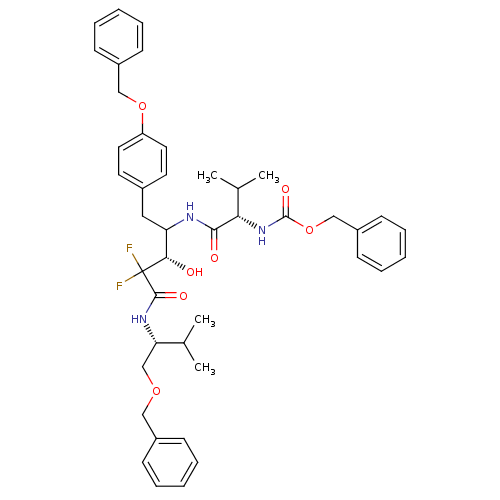
(CHEMBL278084 | {(S)-1-[(S)-1-(4-Benzyloxy-benzyl)-...)Show SMILES CC(C)[C@H](COCc1ccccc1)NC(=O)C(F)(F)[C@@H](O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H51F2N3O7/c1-29(2)37(28-53-25-32-14-8-5-9-15-32)47-41(51)43(44,45)39(49)36(24-31-20-22-35(23-21-31)54-26-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)55-27-34-18-12-7-13-19-34/h5-23,29-30,36-39,49H,24-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36?,37-,38-,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283361

(5-(4-Benzyloxy-phenyl)-2,2-difluoro-4-[(S)-3-methy...)Show SMILES CC(C)[C@H](NC(=O)CCc1cccnc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C38H40F2N4O5/c1-26(2)34(44-33(45)20-17-29-14-9-21-41-23-29)36(47)43-32(35(46)38(39,40)37(48)42-24-28-10-5-3-6-11-28)22-27-15-18-31(19-16-27)49-25-30-12-7-4-8-13-30/h3-16,18-19,21,23,26,32,34H,17,20,22,24-25H2,1-2H3,(H,42,48)(H,43,47)(H,44,45)/t32?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282530
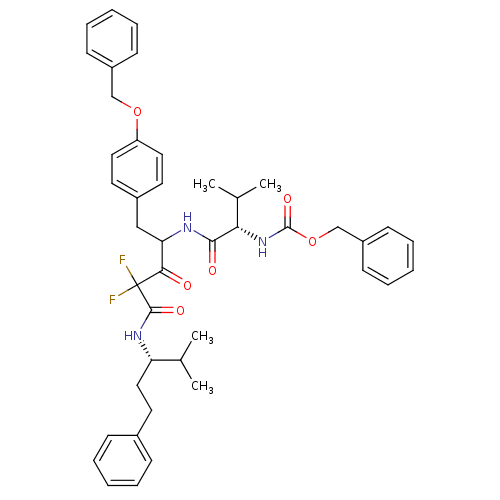
(CHEMBL20135 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-d...)Show SMILES CC(C)[C@H](CCc1ccccc1)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H49F2N3O6/c1-29(2)36(25-22-31-14-8-5-9-15-31)47-41(51)43(44,45)39(49)37(26-32-20-23-35(24-21-32)53-27-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)54-28-34-18-12-7-13-19-34/h5-21,23-24,29-30,36-38H,22,25-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36-,37?,38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283354

(CHEMBL79345 | {(S)-1-[3-(Benzyl-ethyl-carbamoyl)-1...)Show SMILES CCN(Cc1ccccc1)C(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C40H43F2N3O6/c1-4-45(25-30-14-8-5-9-15-30)38(48)40(41,42)36(46)34(24-29-20-22-33(23-21-29)50-26-31-16-10-6-11-17-31)43-37(47)35(28(2)3)44-39(49)51-27-32-18-12-7-13-19-32/h5-23,28,34-35H,4,24-27H2,1-3H3,(H,43,47)(H,44,49)/t34?,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283356

(CHEMBL309464 | {(S)-1-[3-Benzylcarbamoyl-1-(4-benz...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C39H41F2N3O6/c1-27(2)22-34(44-38(48)50-26-31-16-10-5-11-17-31)36(46)43-33(35(45)39(40,41)37(47)42-24-29-12-6-3-7-13-29)23-28-18-20-32(21-19-28)49-25-30-14-8-4-9-15-30/h3-21,27,33-34H,22-26H2,1-2H3,(H,42,47)(H,43,46)(H,44,48)/t33?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282532
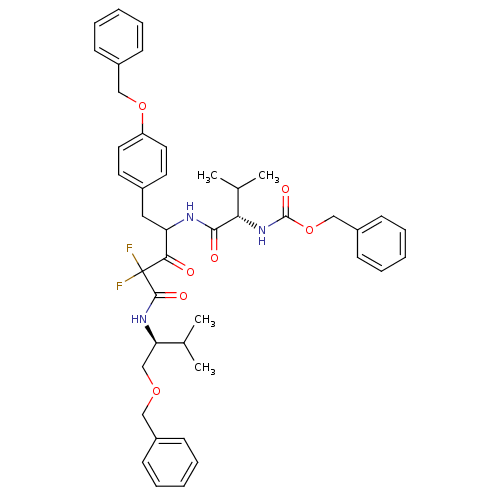
(CHEMBL280398 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3-((...)Show SMILES CC(C)[C@@H](COCc1ccccc1)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H49F2N3O7/c1-29(2)37(28-53-25-32-14-8-5-9-15-32)47-41(51)43(44,45)39(49)36(24-31-20-22-35(23-21-31)54-26-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)55-27-34-18-12-7-13-19-34/h5-23,29-30,36-38H,24-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36?,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283363
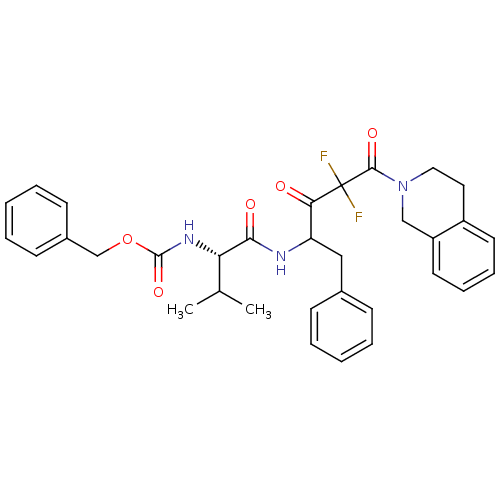
(CHEMBL312809 | {(S)-1-[1-Benzyl-4-(3,4-dihydro-1H-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)N1CCc2ccccc2C1 Show InChI InChI=1S/C33H35F2N3O5/c1-22(2)28(37-32(42)43-21-24-13-7-4-8-14-24)30(40)36-27(19-23-11-5-3-6-12-23)29(39)33(34,35)31(41)38-18-17-25-15-9-10-16-26(25)20-38/h3-16,22,27-28H,17-21H2,1-2H3,(H,36,40)(H,37,42)/t27?,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282533
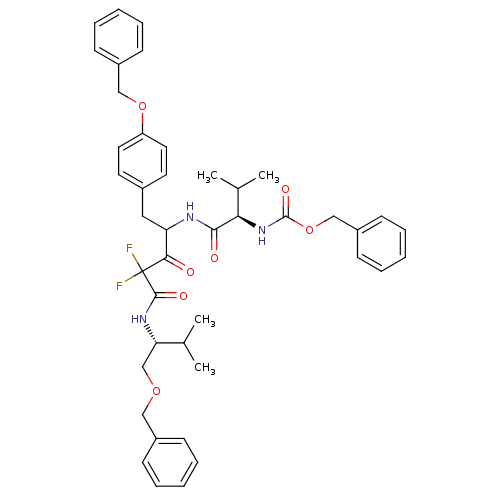
(CHEMBL262702 | {(R)-1-[1-(4-Benzyloxy-benzyl)-3-((...)Show SMILES CC(C)[C@H](COCc1ccccc1)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H49F2N3O7/c1-29(2)37(28-53-25-32-14-8-5-9-15-32)47-41(51)43(44,45)39(49)36(24-31-20-22-35(23-21-31)54-26-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)55-27-34-18-12-7-13-19-34/h5-23,29-30,36-38H,24-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36?,37-,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283353
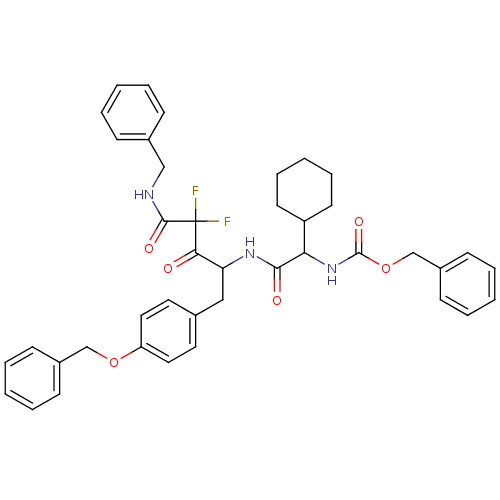
(CHEMBL81135 | {[3-Benzylcarbamoyl-1-(4-benzyloxy-b...)Show SMILES FC(F)(C(=O)NCc1ccccc1)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)C(NC(=O)OCc1ccccc1)C1CCCCC1 Show InChI InChI=1S/C41H43F2N3O6/c42-41(43,39(49)44-26-30-13-5-1-6-14-30)37(47)35(25-29-21-23-34(24-22-29)51-27-31-15-7-2-8-16-31)45-38(48)36(33-19-11-4-12-20-33)46-40(50)52-28-32-17-9-3-10-18-32/h1-3,5-10,13-18,21-24,33,35-36H,4,11-12,19-20,25-28H2,(H,44,49)(H,45,48)(H,46,50) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283360
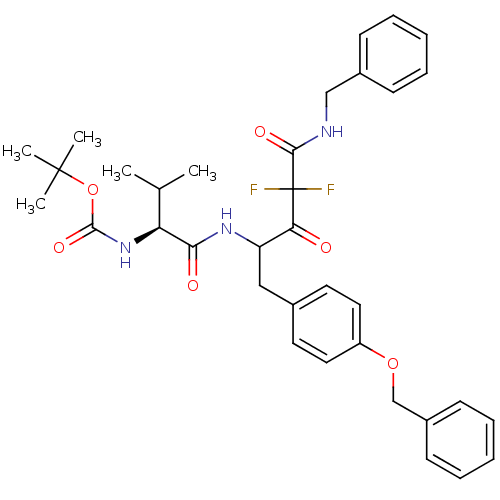
(CHEMBL311259 | {(S)-1-[3-Benzylcarbamoyl-1-(4-benz...)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C35H41F2N3O6/c1-23(2)29(40-33(44)46-34(3,4)5)31(42)39-28(30(41)35(36,37)32(43)38-21-25-12-8-6-9-13-25)20-24-16-18-27(19-17-24)45-22-26-14-10-7-11-15-26/h6-19,23,28-29H,20-22H2,1-5H3,(H,38,43)(H,39,42)(H,40,44)/t28?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282536
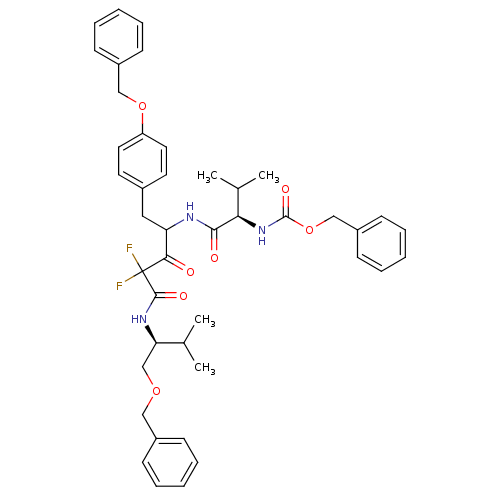
(CHEMBL278823 | {(R)-1-[1-(4-Benzyloxy-benzyl)-3-((...)Show SMILES CC(C)[C@@H](COCc1ccccc1)NC(=O)C(F)(F)C(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C43H49F2N3O7/c1-29(2)37(28-53-25-32-14-8-5-9-15-32)47-41(51)43(44,45)39(49)36(24-31-20-22-35(23-21-31)54-26-33-16-10-6-11-17-33)46-40(50)38(30(3)4)48-42(52)55-27-34-18-12-7-13-19-34/h5-23,29-30,36-38H,24-28H2,1-4H3,(H,46,50)(H,47,51)(H,48,52)/t36?,37-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HIV-1 protease. |
Bioorg Med Chem Lett 4: 1213-1218 (1994)
Article DOI: 10.1016/S0960-894X(01)80332-2
BindingDB Entry DOI: 10.7270/Q24Q7TXS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283365

(5-(4-Benzyloxy-phenyl)-2,2-difluoro-4-((S)-3-methy...)Show SMILES CC(C)[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC(Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C37H39F2N3O6S/c1-26(2)33(42-49(46,47)25-30-16-10-5-11-17-30)35(44)41-32(34(43)37(38,39)36(45)40-23-28-12-6-3-7-13-28)22-27-18-20-31(21-19-27)48-24-29-14-8-4-9-15-29/h3-21,26,32-33,42H,22-25H2,1-2H3,(H,40,45)(H,41,44)/t32?,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inactivation of purified HIV-1 protease in vitro |
Bioorg Med Chem Lett 4: 241-246 (1994)
Article DOI: 10.1016/S0960-894X(01)80122-0
BindingDB Entry DOI: 10.7270/Q2HQ3ZVF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data