

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... | Eur J Med Chem 151: 495-507 (2018) Article DOI: 10.1016/j.ejmech.2018.02.074 BindingDB Entry DOI: 10.7270/Q2NK3HKB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... | Eur J Med Chem 151: 495-507 (2018) Article DOI: 10.1016/j.ejmech.2018.02.074 BindingDB Entry DOI: 10.7270/Q2NK3HKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50370401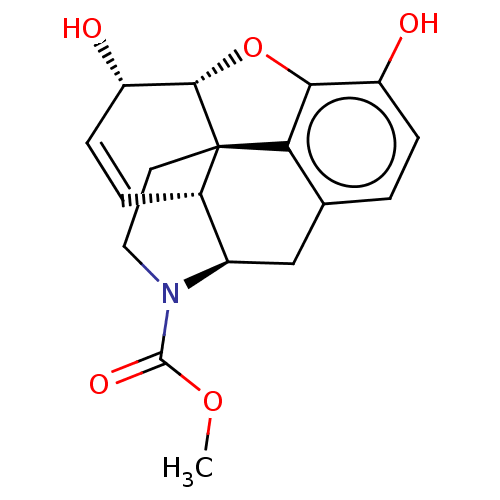 (CHEMBL4168822) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 814 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... | Eur J Med Chem 151: 495-507 (2018) Article DOI: 10.1016/j.ejmech.2018.02.074 BindingDB Entry DOI: 10.7270/Q2NK3HKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50370393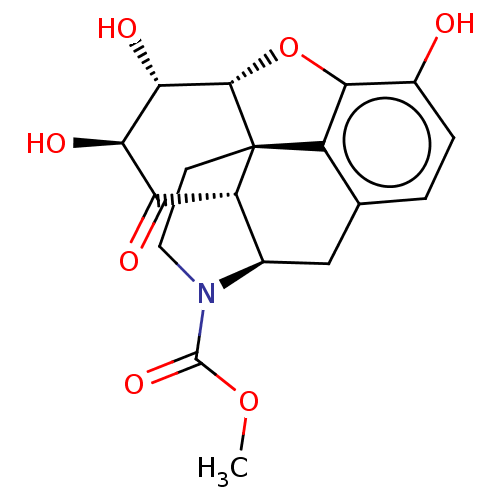 (CHEMBL4168247) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... | Eur J Med Chem 151: 495-507 (2018) Article DOI: 10.1016/j.ejmech.2018.02.074 BindingDB Entry DOI: 10.7270/Q2NK3HKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50370393 (CHEMBL4168247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... | Eur J Med Chem 151: 495-507 (2018) Article DOI: 10.1016/j.ejmech.2018.02.074 BindingDB Entry DOI: 10.7270/Q2NK3HKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50370401 (CHEMBL4168822) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... | Eur J Med Chem 151: 495-507 (2018) Article DOI: 10.1016/j.ejmech.2018.02.074 BindingDB Entry DOI: 10.7270/Q2NK3HKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065646 (CHEMBL3087181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 10 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 30 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 60 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 90 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065640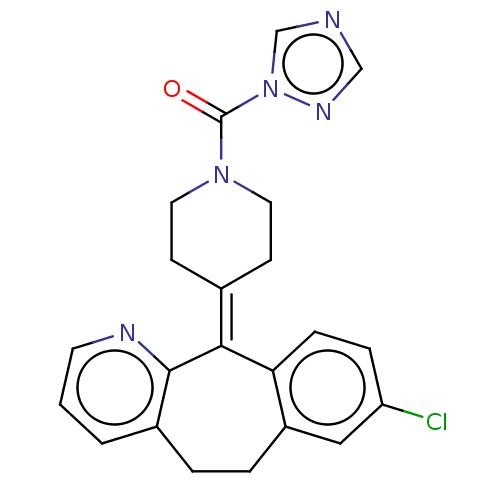 (CHEMBL3401455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065645 (CHEMBL3402827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human ABHD6 containing pCMV6-AC-hABHD6 transfected into HEK293 cells | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065639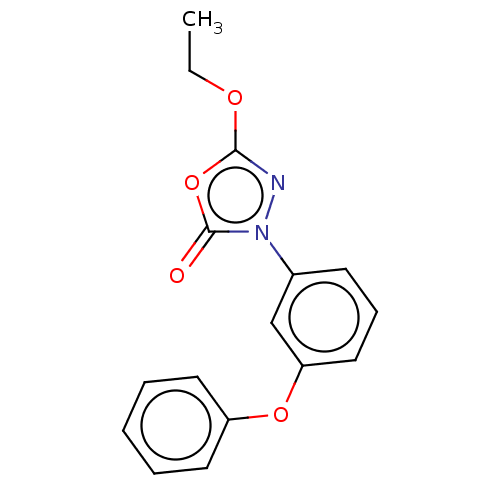 (CHEMBL3402828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 10 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065639 (CHEMBL3402828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 30 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065642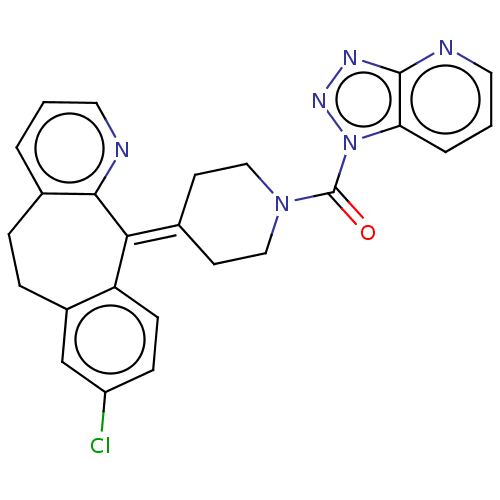 (CHEMBL3401457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065639 (CHEMBL3402828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 60 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidylserine lipase ABHD12 (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human ABHD12 containing pCMV6-XL4-hABHD12 transfected into HEK293 cells | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065641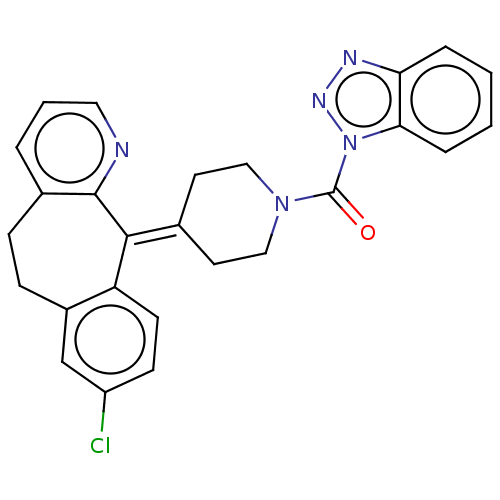 (CHEMBL3401456) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065640 (CHEMBL3401455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 10 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065640 (CHEMBL3401455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 30 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065640 (CHEMBL3401455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 60 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065640 (CHEMBL3401455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 90 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065643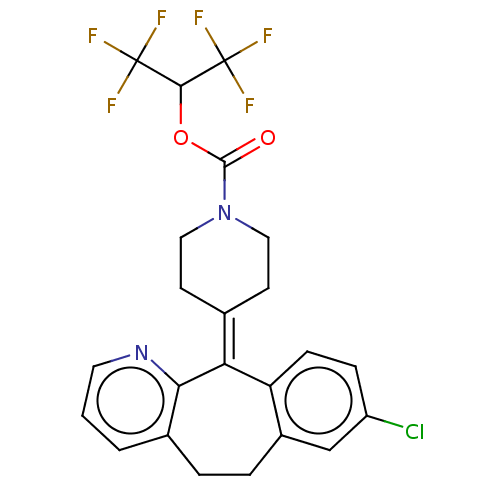 (CHEMBL3402819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065639 (CHEMBL3402828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid after 90 mins by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50065645 (CHEMBL3402827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human ABHD6 containing pCMV6-AC-hABHD6 transfected into HEK293 cells | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50065640 (CHEMBL3401455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human ABHD6 containing pCMV6-AC-hABHD6 transfected into HEK293 cells | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50065644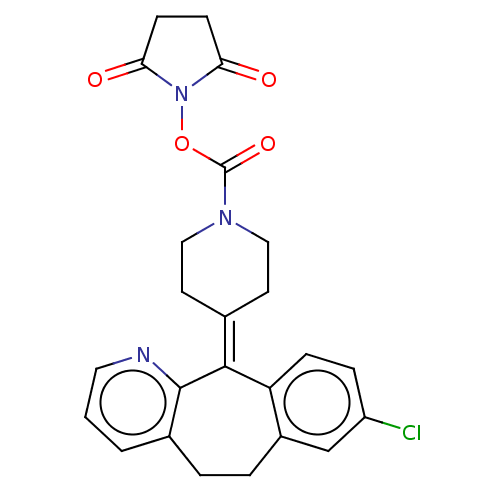 (CHEMBL3402820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 2-arachidonoylglycerol substrate assessed as arachidonic acid by HPLC analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50065646 (CHEMBL3087181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human FAAH using [ethanolamine 1-3H] substrate assessed as radioactivity by liquid scintillation counting analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50065645 (CHEMBL3402827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human FAAH using [ethanolamine 1-3H] substrate assessed as radioactivity by liquid scintillation counting analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50065640 (CHEMBL3401455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human FAAH using [ethanolamine 1-3H] substrate assessed as radioactivity by liquid scintillation counting analysis | Bioorg Med Chem Lett 25: 1436-42 (2015) Article DOI: 10.1016/j.bmcl.2015.02.037 BindingDB Entry DOI: 10.7270/Q20Z74Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||