

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM199186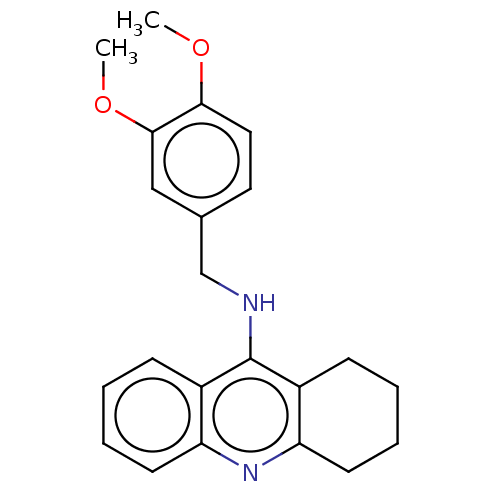 (N-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydroacridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199199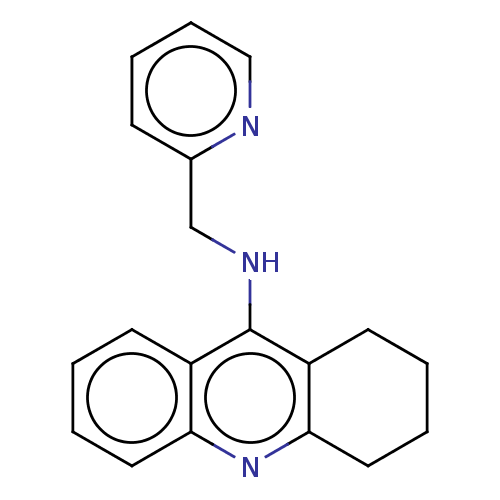 (N-(Pyridin-2-ylmethyl)-1,2,3,4-tetrahydroacridin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199196 (N-(3,4-Dimethoxyphenethyl)-1,2,3,4-tetrahydroacrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199184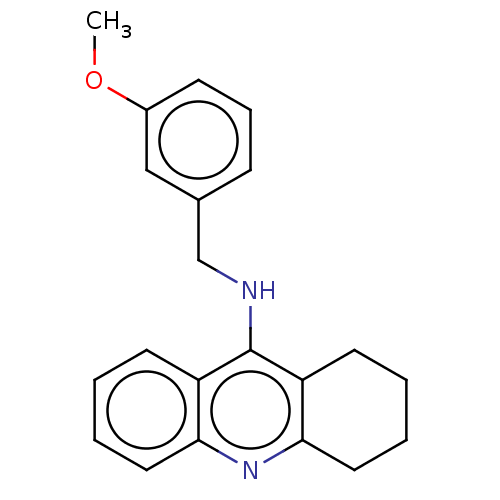 (N-(3-Methoxybenzyl)-1,2,3,4-tetrahydroacridin-9-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199201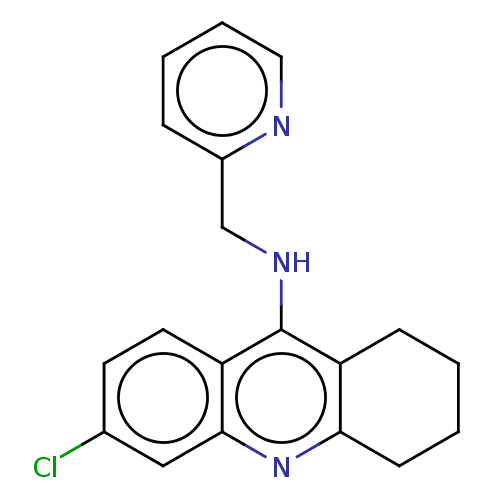 (6-Chloro-N-(pyridin-2-ylmethyl)-1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199183 (N-(2-Methoxybenzyl)-1,2,3,4-tetrahydroacridin-9-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199200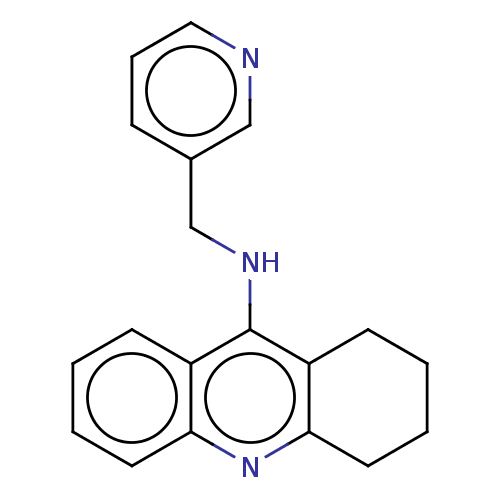 (N-(Pyridin-3-ylmethyl)-1,2,3,4-tetrahydroacridin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199185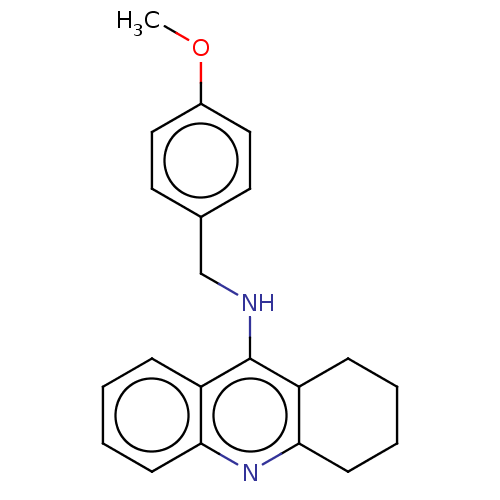 (N-(4-Methoxybenzyl)-1,2,3,4-tetrahydroacridin-9-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199202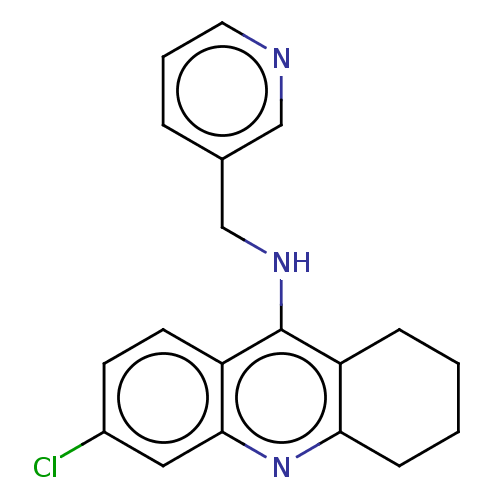 (6-Chloro-N-(pyridin-3-ylmethyl)-1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199182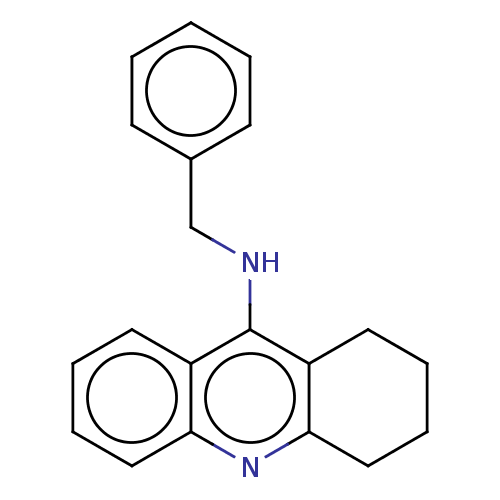 (N-benzyl-1,2,3,4-tetrahydroacridin-9-amine (8a)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387090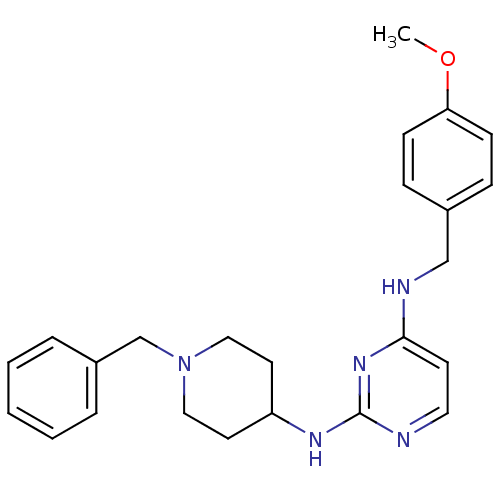 (CHEMBL2047229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387094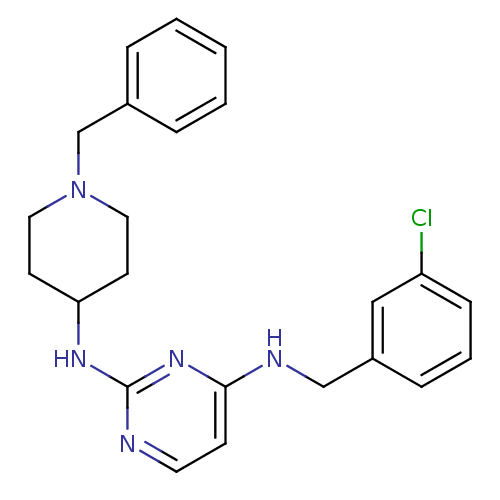 (CHEMBL2047224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199197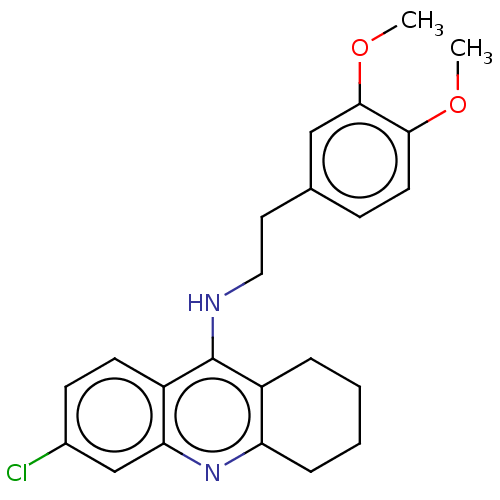 (6-Chloro-N-(3,4-dimethoxyphenethyl)-1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199188 (6-Chloro-N-(3-methoxybenzyl)-1,2,3,4-tetrahydroacr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199189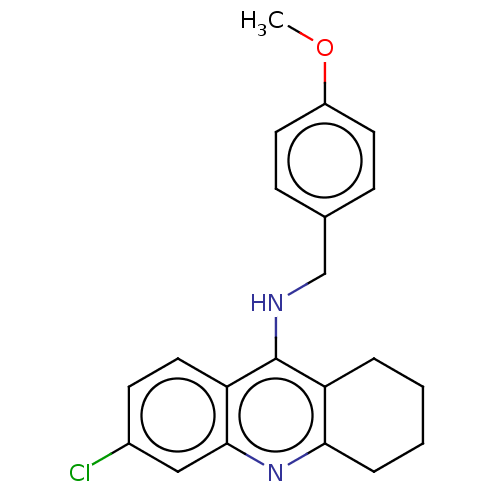 (6-Chloro-N-(4-methoxybenzyl)-1,2,3,4-tetrahydroacr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387092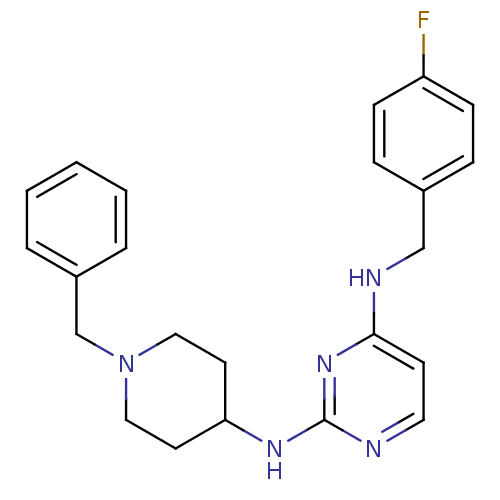 (CHEMBL2047227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199190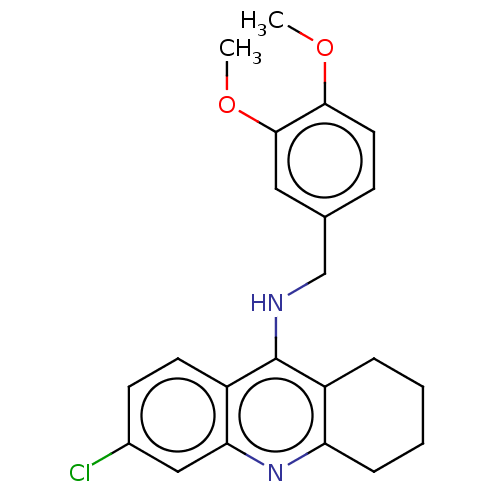 (6-Chloro-N-(3,4-dimethoxybenzyl)-1,2,3,4-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199195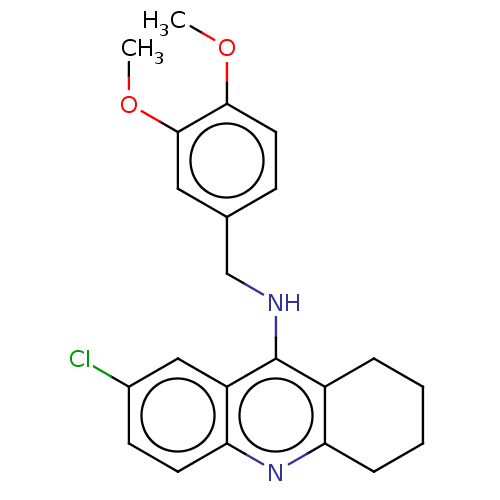 (7-Chloro-N-(3,4-dimethoxybenzyl)-1,2,3,4-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387088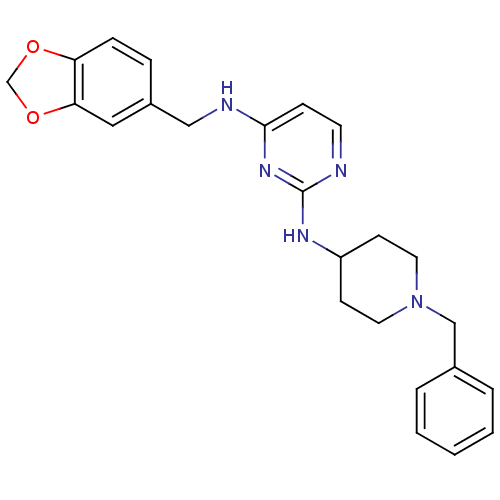 (CHEMBL2047375) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199190 (6-Chloro-N-(3,4-dimethoxybenzyl)-1,2,3,4-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199201 (6-Chloro-N-(pyridin-2-ylmethyl)-1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387095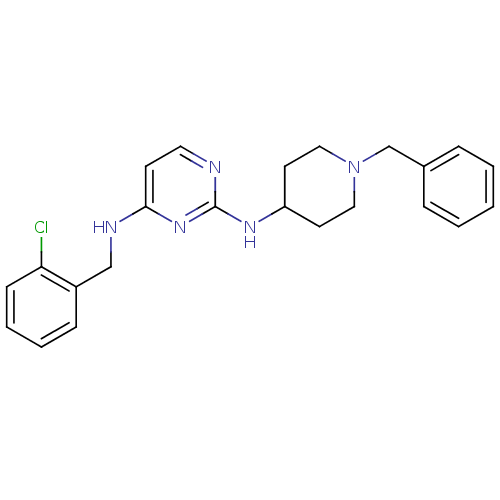 (CHEMBL2047223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8997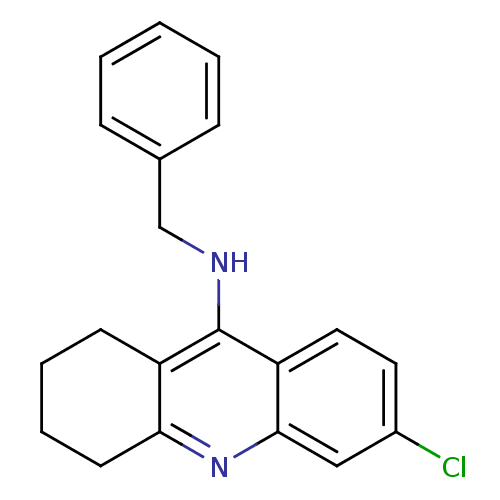 (N-Benzyl-6-chloro-1,2,3,4-tetrahydroacridin-9-amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199197 (6-Chloro-N-(3,4-dimethoxyphenethyl)-1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199193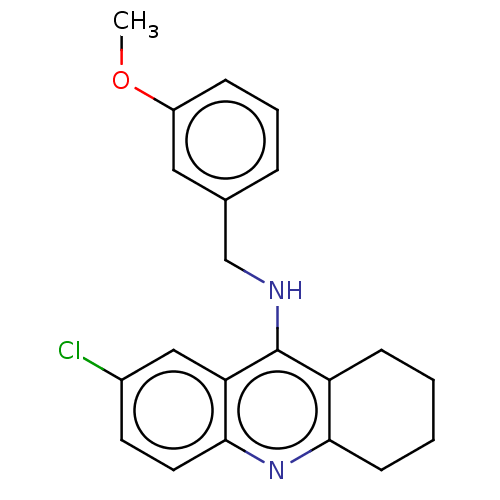 (7-Chloro-N-(3-methoxybenzyl)-1,2,3,4-tetrahydroacr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199185 (N-(4-Methoxybenzyl)-1,2,3,4-tetrahydroacridin-9-am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199186 (N-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydroacridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50387092 (CHEMBL2047227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199187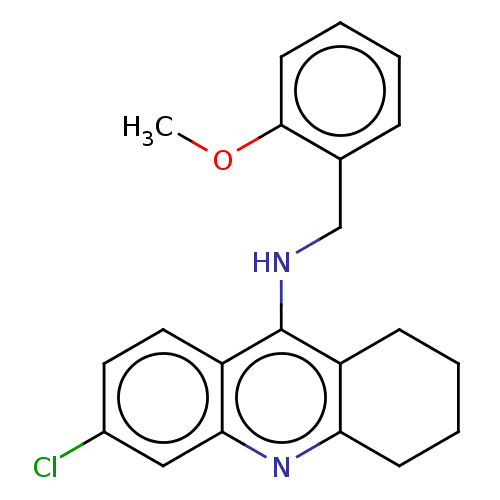 (6-Chloro-N-(2-methoxybenzyl)-1,2,3,4-tetrahydroacr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50387095 (CHEMBL2047223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50387094 (CHEMBL2047224) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50387091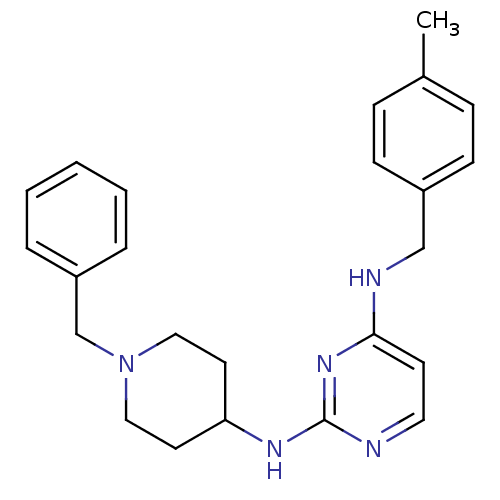 (CHEMBL2047228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199191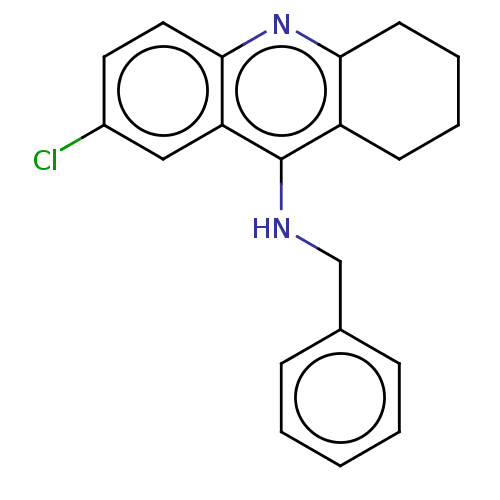 (N-Benzyl-7-chloro-1,2,3,4-tetrahydroacridin-9-amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50387093 (CHEMBL2047225) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199187 (6-Chloro-N-(2-methoxybenzyl)-1,2,3,4-tetrahydroacr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199198 (7-Chloro-N-(3,4-dimethoxyphenethyl)-1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50387093 (CHEMBL2047225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199184 (N-(3-Methoxybenzyl)-1,2,3,4-tetrahydroacridin-9-am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199194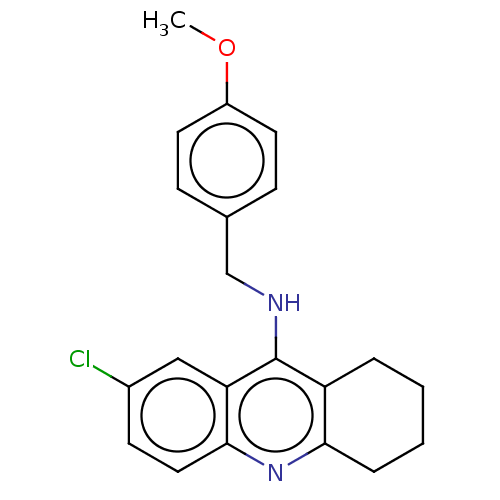 (7-Chloro-N-(4-methoxybenzyl)-1,2,3,4-tetrahydroacr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50387088 (CHEMBL2047375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE using butyrylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199200 (N-(Pyridin-3-ylmethyl)-1,2,3,4-tetrahydroacridin-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |