 Found 341 hits with Last Name = 'vaudry' and Initial = 'h'
Found 341 hits with Last Name = 'vaudry' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002726
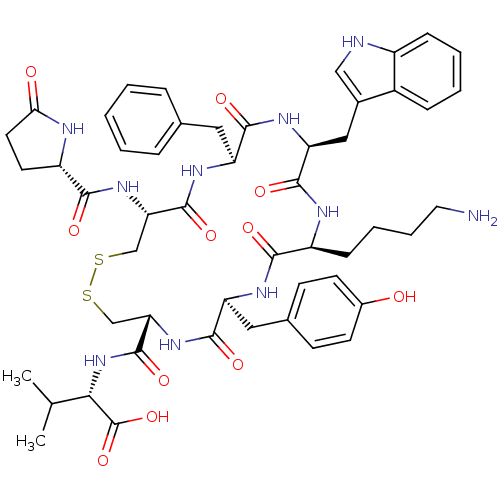
(CHEMBL2371933)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H]2CCC(=O)N2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C51H64N10O11S2/c1-28(2)43(51(71)72)61-50(70)41-27-74-73-26-40(59-45(65)36-19-20-42(63)54-36)49(69)57-37(22-29-10-4-3-5-11-29)46(66)58-39(24-31-25-53-34-13-7-6-12-33(31)34)48(68)55-35(14-8-9-21-52)44(64)56-38(47(67)60-41)23-30-15-17-32(62)18-16-30/h3-7,10-13,15-18,25,28,35-41,43,53,62H,8-9,14,19-24,26-27,52H2,1-2H3,(H,54,63)(H,55,68)(H,56,64)(H,57,69)(H,58,66)(H,59,65)(H,60,67)(H,61,70)(H,71,72)/t35-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002718
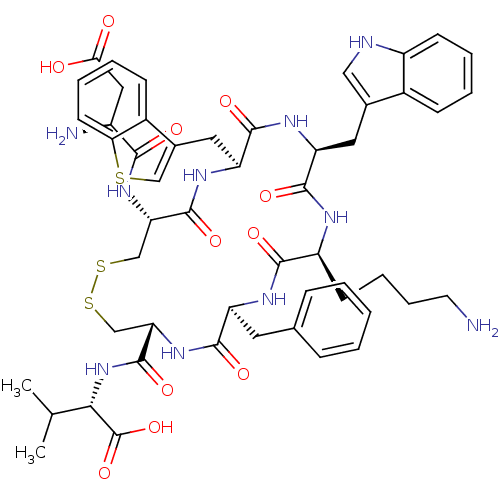
(CHEMBL385281)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2csc3ccccc23)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C52H64N10O11S3/c1-28(2)44(52(72)73)62-51(71)41-27-76-75-26-40(60-45(65)34(54)23-43(63)64)50(70)59-39(22-31-25-74-42-18-9-7-15-33(31)42)49(69)58-38(21-30-24-55-35-16-8-6-14-32(30)35)48(68)56-36(17-10-11-19-53)46(66)57-37(47(67)61-41)20-29-12-4-3-5-13-29/h3-9,12-16,18,24-25,28,34,36-41,44,55H,10-11,17,19-23,26-27,53-54H2,1-2H3,(H,56,68)(H,57,66)(H,58,69)(H,59,70)(H,60,65)(H,61,67)(H,62,71)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,44-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50320463
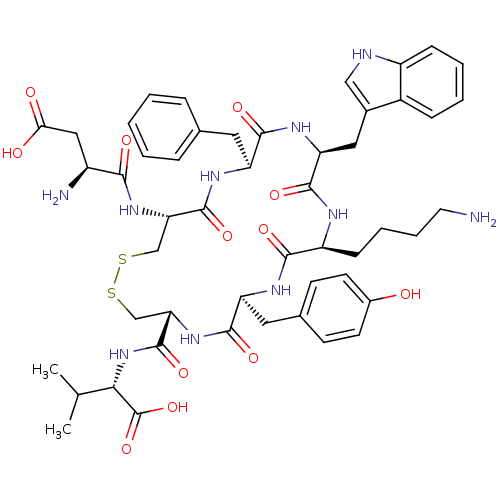
(CHEMBL218994 | D[CFWKYC]V | H-Asp-Cys-Phe-Trp-Lys-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H64N10O12S2/c1-27(2)42(50(71)72)60-49(70)40-26-74-73-25-39(58-43(64)33(52)23-41(62)63)48(69)56-36(20-28-10-4-3-5-11-28)45(66)57-38(22-30-24-53-34-13-7-6-12-32(30)34)47(68)54-35(14-8-9-19-51)44(65)55-37(46(67)59-40)21-29-15-17-31(61)18-16-29/h3-7,10-13,15-18,24,27,33,35-40,42,53,61H,8-9,14,19-23,25-26,51-52H2,1-2H3,(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,64)(H,59,67)(H,60,70)(H,62,63)(H,71,72)/t33-,35-,36-,37-,38-,39-,40-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002725
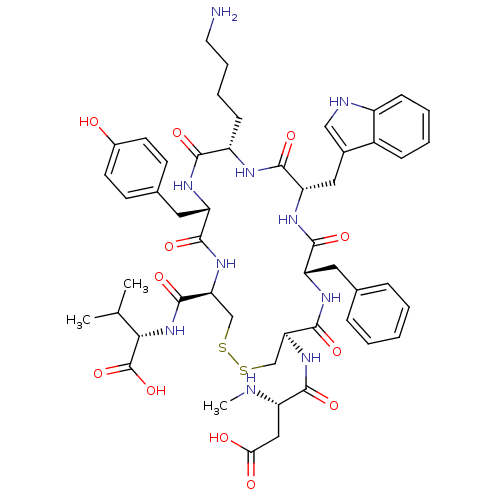
(CHEMBL374468)Show SMILES CN[C@@H](CC(O)=O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)61-50(71)41-27-75-74-26-40(59-45(66)36(53-3)24-42(63)64)49(70)57-37(21-29-11-5-4-6-12-29)46(67)58-39(23-31-25-54-34-14-8-7-13-33(31)34)48(69)55-35(15-9-10-20-52)44(65)56-38(47(68)60-41)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,35-41,43,53-54,62H,9-10,15,20-24,26-27,52H2,1-3H3,(H,55,69)(H,56,65)(H,57,70)(H,58,67)(H,59,66)(H,60,68)(H,61,71)(H,63,64)(H,72,73)/t35-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002728
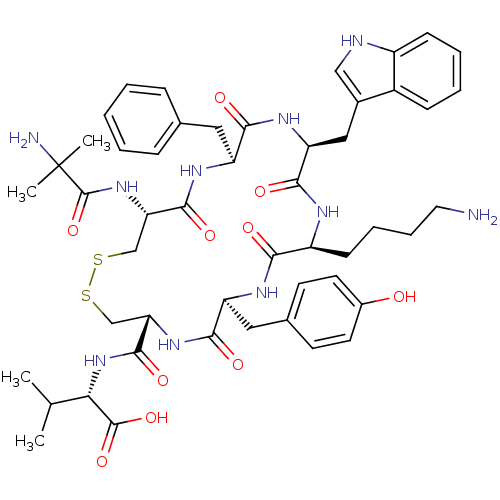
(CHEMBL218825)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)C(C)(C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)41(48(68)69)60-47(67)39-26-71-72-27-40(59-49(70)50(3,4)52)46(66)56-36(22-29-12-6-5-7-13-29)43(63)57-38(24-31-25-53-34-15-9-8-14-33(31)34)45(65)54-35(16-10-11-21-51)42(62)55-37(44(64)58-39)23-30-17-19-32(61)20-18-30/h5-9,12-15,17-20,25,28,35-41,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,62)(H,56,66)(H,57,63)(H,58,64)(H,59,70)(H,60,67)(H,68,69)/t35-,36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002723

(CHEMBL265166)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)c(c2)[N+]([O-])=O)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H63N11O14S2/c1-26(2)42(50(72)73)60-49(71)38-25-77-76-24-37(58-43(65)31(52)22-41(63)64)48(70)56-34(18-27-10-4-3-5-11-27)45(67)57-36(21-29-23-53-32-13-7-6-12-30(29)32)47(69)54-33(14-8-9-17-51)44(66)55-35(46(68)59-38)19-28-15-16-40(62)39(20-28)61(74)75/h3-7,10-13,15-16,20,23,26,31,33-38,42,53,62H,8-9,14,17-19,21-22,24-25,51-52H2,1-2H3,(H,54,69)(H,55,66)(H,56,70)(H,57,67)(H,58,65)(H,59,68)(H,60,71)(H,63,64)(H,72,73)/t31-,33-,34-,35-,36-,37-,38-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002717
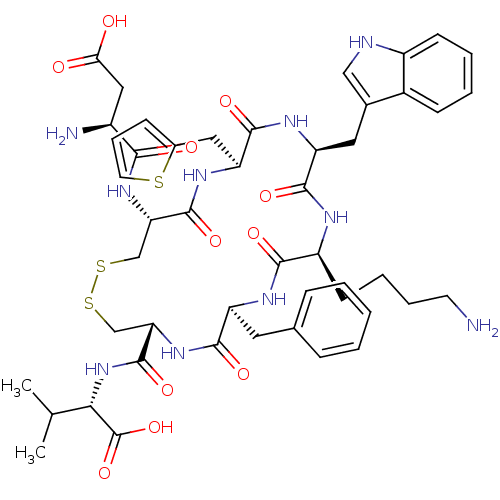
(CHEMBL216712)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2cccs2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C48H62N10O11S3/c1-26(2)40(48(68)69)58-47(67)38-25-72-71-24-37(56-41(61)31(50)22-39(59)60)46(66)55-36(21-29-13-10-18-70-29)45(65)54-35(20-28-23-51-32-15-7-6-14-30(28)32)44(64)52-33(16-8-9-17-49)42(62)53-34(43(63)57-38)19-27-11-4-3-5-12-27/h3-7,10-15,18,23,26,31,33-38,40,51H,8-9,16-17,19-22,24-25,49-50H2,1-2H3,(H,52,64)(H,53,62)(H,54,65)(H,55,66)(H,56,61)(H,57,63)(H,58,67)(H,59,60)(H,68,69)/t31-,33-,34-,35-,36-,37-,38-,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002727
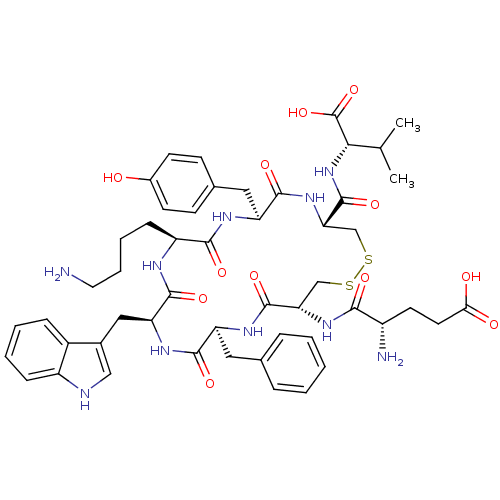
(CHEMBL266651)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)61-50(71)41-27-75-74-26-40(59-44(65)34(53)19-20-42(63)64)49(70)57-37(22-29-10-4-3-5-11-29)46(67)58-39(24-31-25-54-35-13-7-6-12-33(31)35)48(69)55-36(14-8-9-21-52)45(66)56-38(47(68)60-41)23-30-15-17-32(62)18-16-30/h3-7,10-13,15-18,25,28,34,36-41,43,54,62H,8-9,14,19-24,26-27,52-53H2,1-2H3,(H,55,69)(H,56,66)(H,57,70)(H,58,67)(H,59,65)(H,60,68)(H,61,71)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM82253
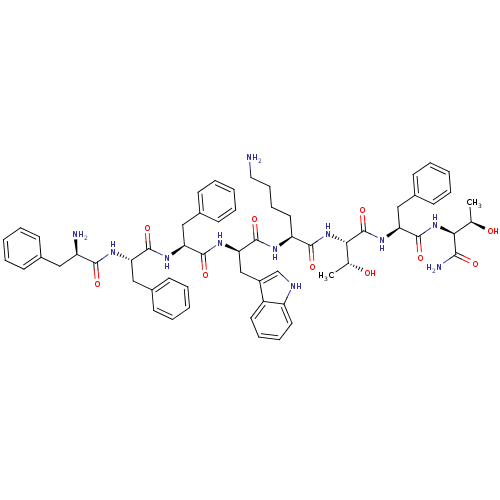
(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50170158

(1-(2-Methoxy-phenyl)-4-(1-o-tolyl-1H-pyrrol-3-ylme...)Show InChI InChI=1S/C23H27N3O/c1-19-7-3-4-8-21(19)26-12-11-20(18-26)17-24-13-15-25(16-14-24)22-9-5-6-10-23(22)27-2/h3-12,18H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50170158

(1-(2-Methoxy-phenyl)-4-(1-o-tolyl-1H-pyrrol-3-ylme...)Show InChI InChI=1S/C23H27N3O/c1-19-7-3-4-8-21(19)26-12-11-20(18-26)17-24-13-15-25(16-14-24)22-9-5-6-10-23(22)27-2/h3-12,18H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against Human 5-HT7R expressed in sf9 cells |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50170156

(2-(3-((4-(2-methoxyphenyl)piperazin-1-yl)methyl)-1...)Show InChI InChI=1S/C23H24N4O/c1-28-23-9-5-4-8-22(23)26-14-12-25(13-15-26)17-19-10-11-27(18-19)21-7-3-2-6-20(21)16-24/h2-11,18H,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50170156

(2-(3-((4-(2-methoxyphenyl)piperazin-1-yl)methyl)-1...)Show InChI InChI=1S/C23H24N4O/c1-28-23-9-5-4-8-22(23)26-14-12-25(13-15-26)17-19-10-11-27(18-19)21-7-3-2-6-20(21)16-24/h2-11,18H,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against Human 5-HT7R expressed in sf9 cells |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50210122

(CHEMBL234881 | [2-(2',6'-dimethyl-biphenyl-3-yl)-e...)Show InChI InChI=1S/C18H23N/c1-14-7-5-8-15(2)18(14)17-10-6-9-16(13-17)11-12-19(3)4/h5-10,13H,11-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50210124

(2-(2'-methyl-biphenyl-3-yl)-ethylamine | CHEMBL234...)Show InChI InChI=1S/C15H17N/c1-12-5-2-3-8-15(12)14-7-4-6-13(11-14)9-10-16/h2-8,11H,9-10,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50210123

(CHEMBL395870 | N,N-dimethyl-2-(2'-methylbiphenyl-3...)Show InChI InChI=1S/C17H21N/c1-14-7-4-5-10-17(14)16-9-6-8-15(13-16)11-12-18(2)3/h4-10,13H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50210126
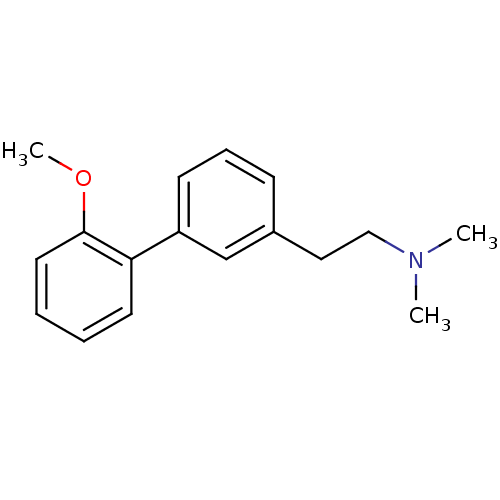
(CHEMBL232792 | [2-(2'-methoxy-biphenyl-3-yl)-ethyl...)Show InChI InChI=1S/C17H21NO/c1-18(2)12-11-14-7-6-8-15(13-14)16-9-4-5-10-17(16)19-3/h4-10,13H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50210125
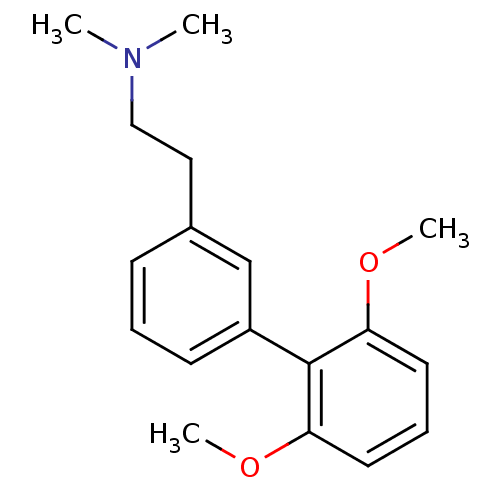
(CHEMBL232791 | [2-(2',6'-dimethoxy-biphenyl-3-yl)-...)Show InChI InChI=1S/C18H23NO2/c1-19(2)12-11-14-7-5-8-15(13-14)18-16(20-3)9-6-10-17(18)21-4/h5-10,13H,11-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002716
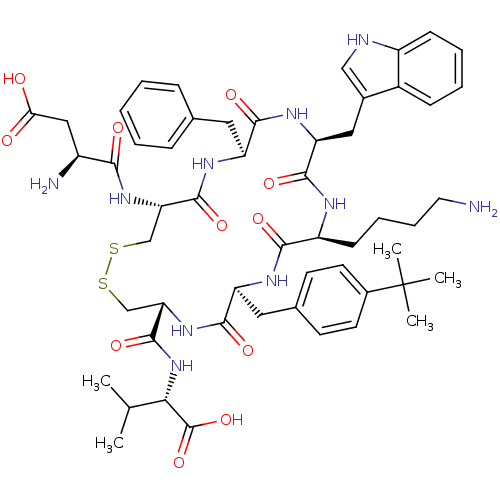
(CHEMBL383996)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(cc2)C(C)(C)C)C(=O)N1)C(O)=O Show InChI InChI=1S/C54H72N10O11S2/c1-30(2)45(53(74)75)64-52(73)43-29-77-76-28-42(62-46(67)36(56)26-44(65)66)51(72)60-39(23-31-13-7-6-8-14-31)48(69)61-41(25-33-27-57-37-16-10-9-15-35(33)37)50(71)58-38(17-11-12-22-55)47(68)59-40(49(70)63-43)24-32-18-20-34(21-19-32)54(3,4)5/h6-10,13-16,18-21,27,30,36,38-43,45,57H,11-12,17,22-26,28-29,55-56H2,1-5H3,(H,58,71)(H,59,68)(H,60,72)(H,61,69)(H,62,67)(H,63,70)(H,64,73)(H,65,66)(H,74,75)/t36-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50170158

(1-(2-Methoxy-phenyl)-4-(1-o-tolyl-1H-pyrrol-3-ylme...)Show InChI InChI=1S/C23H27N3O/c1-19-7-3-4-8-21(19)26-12-11-20(18-26)17-24-13-15-25(16-14-24)22-9-5-6-10-23(22)27-2/h3-12,18H,13-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-HT1A receptor |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50170158

(1-(2-Methoxy-phenyl)-4-(1-o-tolyl-1H-pyrrol-3-ylme...)Show InChI InChI=1S/C23H27N3O/c1-19-7-3-4-8-21(19)26-12-11-20(18-26)17-24-13-15-25(16-14-24)22-9-5-6-10-23(22)27-2/h3-12,18H,13-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity to rat 5HT1A |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002719
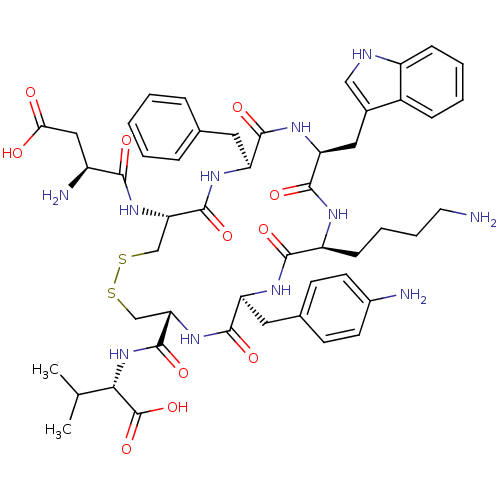
(CHEMBL412179)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(N)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H65N11O11S2/c1-27(2)42(50(71)72)61-49(70)40-26-74-73-25-39(59-43(64)33(53)23-41(62)63)48(69)57-36(20-28-10-4-3-5-11-28)45(66)58-38(22-30-24-54-34-13-7-6-12-32(30)34)47(68)55-35(14-8-9-19-51)44(65)56-37(46(67)60-40)21-29-15-17-31(52)18-16-29/h3-7,10-13,15-18,24,27,33,35-40,42,54H,8-9,14,19-23,25-26,51-53H2,1-2H3,(H,55,68)(H,56,65)(H,57,69)(H,58,66)(H,59,64)(H,60,67)(H,61,70)(H,62,63)(H,71,72)/t33-,35-,36-,37-,38-,39-,40-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50170159
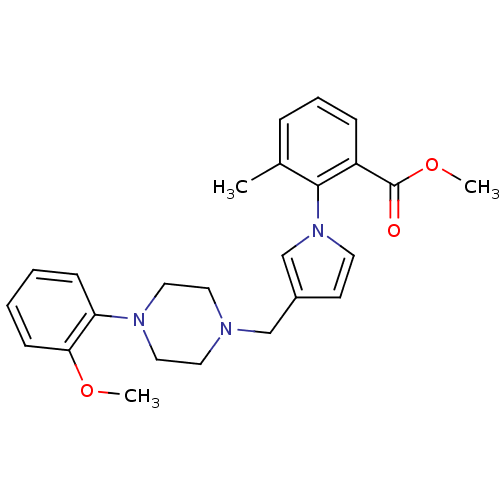
(2-{3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-p...)Show SMILES COC(=O)c1cccc(C)c1-n1ccc(CN2CCN(CC2)c2ccccc2OC)c1 Show InChI InChI=1S/C25H29N3O3/c1-19-7-6-8-21(25(29)31-3)24(19)28-12-11-20(18-28)17-26-13-15-27(16-14-26)22-9-4-5-10-23(22)30-2/h4-12,18H,13-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against Human 5-HT7R expressed in sf9 cells |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50170159

(2-{3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-p...)Show SMILES COC(=O)c1cccc(C)c1-n1ccc(CN2CCN(CC2)c2ccccc2OC)c1 Show InChI InChI=1S/C25H29N3O3/c1-19-7-6-8-21(25(29)31-3)24(19)28-12-11-20(18-28)17-26-13-15-27(16-14-26)22-9-4-5-10-23(22)30-2/h4-12,18H,13-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 in sf9 cells |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50170159

(2-{3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-p...)Show SMILES COC(=O)c1cccc(C)c1-n1ccc(CN2CCN(CC2)c2ccccc2OC)c1 Show InChI InChI=1S/C25H29N3O3/c1-19-7-6-8-21(25(29)31-3)24(19)28-12-11-20(18-28)17-26-13-15-27(16-14-26)22-9-4-5-10-23(22)30-2/h4-12,18H,13-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-HT1A receptor |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50170159

(2-{3-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-p...)Show SMILES COC(=O)c1cccc(C)c1-n1ccc(CN2CCN(CC2)c2ccccc2OC)c1 Show InChI InChI=1S/C25H29N3O3/c1-19-7-6-8-21(25(29)31-3)24(19)28-12-11-20(18-28)17-26-13-15-27(16-14-26)22-9-4-5-10-23(22)30-2/h4-12,18H,13-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity to rat 5HT1A |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002715

(CHEMBL376223)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H69IN10O12S2/c1-26(2)42(50(72)73)61-49(71)39-25-75-74-24-38(59-43(65)32(53)22-41(63)64)48(70)57-35(19-27-10-4-3-5-11-27)45(67)58-37(21-29-23-54-33-13-7-6-12-30(29)33)47(69)55-34(14-8-9-17-52)44(66)56-36(46(68)60-39)20-28-15-16-40(62)31(51)18-28/h6-7,12-13,15-16,18,23,26-27,32,34-39,42,54,62H,3-5,8-11,14,17,19-22,24-25,52-53H2,1-2H3,(H,55,69)(H,56,66)(H,57,70)(H,58,67)(H,59,65)(H,60,68)(H,61,71)(H,63,64)(H,72,73)/t32-,34-,35-,36-,37-,38-,39-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50170156

(2-(3-((4-(2-methoxyphenyl)piperazin-1-yl)methyl)-1...)Show InChI InChI=1S/C23H24N4O/c1-28-23-9-5-4-8-22(23)26-14-12-25(13-15-26)17-19-10-11-27(18-19)21-7-3-2-6-20(21)16-24/h2-11,18H,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-HT7R expressed in HEK-293 cells |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50170158

(1-(2-Methoxy-phenyl)-4-(1-o-tolyl-1H-pyrrol-3-ylme...)Show InChI InChI=1S/C23H27N3O/c1-19-7-3-4-8-21(19)26-12-11-20(18-26)17-24-13-15-25(16-14-24)22-9-5-6-10-23(22)27-2/h3-12,18H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-HT7R expressed in HEK-293 cells |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002720
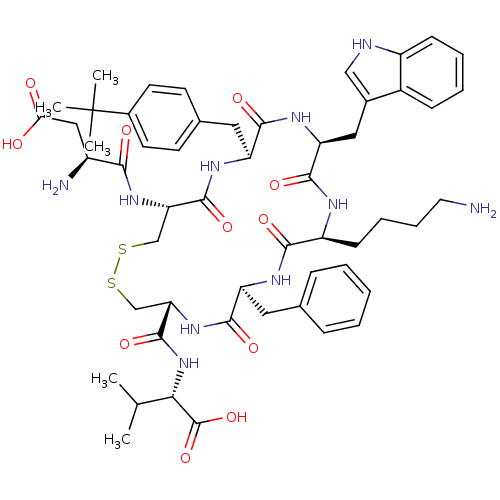
(CHEMBL219356)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccc(cc2)C(C)(C)C)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C54H72N10O11S2/c1-30(2)45(53(74)75)64-52(73)43-29-77-76-28-42(62-46(67)36(56)26-44(65)66)51(72)60-40(24-32-18-20-34(21-19-32)54(3,4)5)48(69)61-41(25-33-27-57-37-16-10-9-15-35(33)37)50(71)58-38(17-11-12-22-55)47(68)59-39(49(70)63-43)23-31-13-7-6-8-14-31/h6-10,13-16,18-21,27,30,36,38-43,45,57H,11-12,17,22-26,28-29,55-56H2,1-5H3,(H,58,71)(H,59,68)(H,60,72)(H,61,69)(H,62,67)(H,63,70)(H,64,73)(H,65,66)(H,74,75)/t36-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM84629
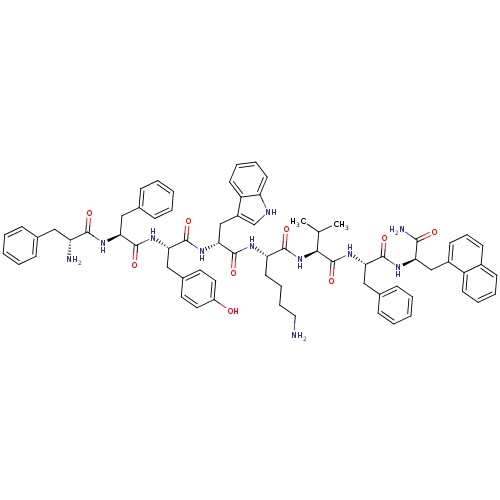
(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50170156

(2-(3-((4-(2-methoxyphenyl)piperazin-1-yl)methyl)-1...)Show InChI InChI=1S/C23H24N4O/c1-28-23-9-5-4-8-22(23)26-14-12-25(13-15-26)17-19-10-11-27(18-19)21-7-3-2-6-20(21)16-24/h2-11,18H,12-15,17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-HT1A receptor |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50170156

(2-(3-((4-(2-methoxyphenyl)piperazin-1-yl)methyl)-1...)Show InChI InChI=1S/C23H24N4O/c1-28-23-9-5-4-8-22(23)26-14-12-25(13-15-26)17-19-10-11-27(18-19)21-7-3-2-6-20(21)16-24/h2-11,18H,12-15,17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity to rat 5HT1A |
Bioorg Med Chem Lett 17: 3018-22 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.054
BindingDB Entry DOI: 10.7270/Q2B857T1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM85080
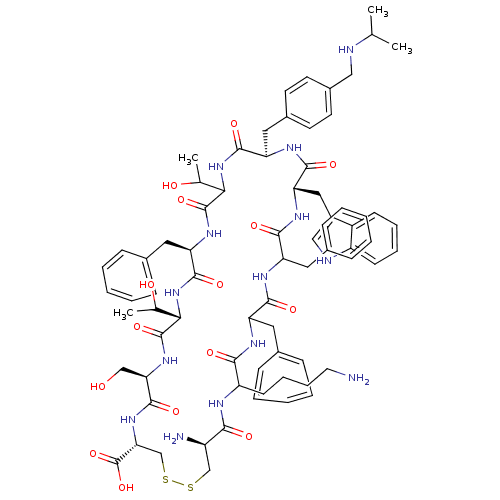
(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002724
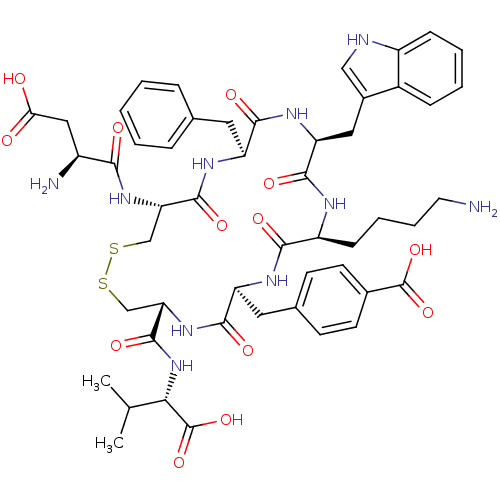
(CHEMBL428990)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(cc2)C(O)=O)C(=O)N1)C(O)=O Show InChI InChI=1S/C51H64N10O13S2/c1-27(2)42(51(73)74)61-49(70)40-26-76-75-25-39(59-43(64)33(53)23-41(62)63)48(69)57-36(20-28-10-4-3-5-11-28)45(66)58-38(22-31-24-54-34-13-7-6-12-32(31)34)47(68)55-35(14-8-9-19-52)44(65)56-37(46(67)60-40)21-29-15-17-30(18-16-29)50(71)72/h3-7,10-13,15-18,24,27,33,35-40,42,54H,8-9,14,19-23,25-26,52-53H2,1-2H3,(H,55,68)(H,56,65)(H,57,69)(H,58,66)(H,59,64)(H,60,67)(H,61,70)(H,62,63)(H,71,72)(H,73,74)/t33-,35-,36-,37-,38-,39-,40-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM84629

(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50170157
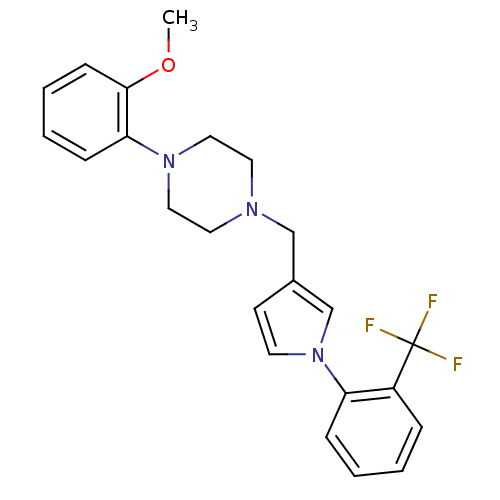
(1-(2-Methoxy-phenyl)-4-[1-(2-trifluoromethyl-pheny...)Show SMILES COc1ccccc1N1CCN(Cc2ccn(c2)-c2ccccc2C(F)(F)F)CC1 Show InChI InChI=1S/C23H24F3N3O/c1-30-22-9-5-4-8-21(22)28-14-12-27(13-15-28)16-18-10-11-29(17-18)20-7-3-2-6-19(20)23(24,25)26/h2-11,17H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-HT7R expressed in HEK-293 cells |
Bioorg Med Chem Lett 15: 3753-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.059
BindingDB Entry DOI: 10.7270/Q2BV7G51 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002722
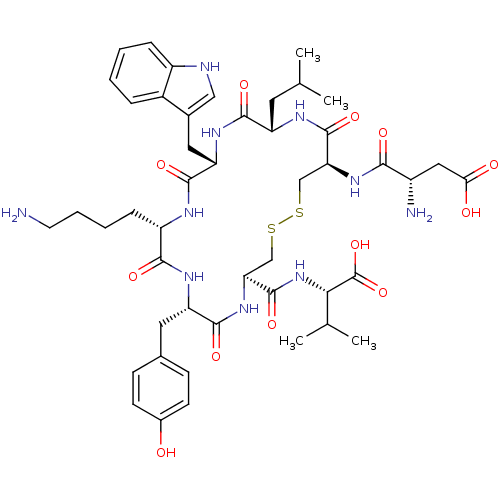
(CHEMBL415851)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O Show InChI InChI=1S/C47H66N10O12S2/c1-24(2)17-33-42(63)54-35(19-27-21-50-31-10-6-5-9-29(27)31)44(65)51-32(11-7-8-16-48)41(62)53-34(18-26-12-14-28(58)15-13-26)43(64)56-37(46(67)57-39(25(3)4)47(68)69)23-71-70-22-36(45(66)52-33)55-40(61)30(49)20-38(59)60/h5-6,9-10,12-15,21,24-25,30,32-37,39,50,58H,7-8,11,16-20,22-23,48-49H2,1-4H3,(H,51,65)(H,52,66)(H,53,62)(H,54,63)(H,55,61)(H,56,64)(H,57,67)(H,59,60)(H,68,69)/t30-,32-,33-,34-,35-,36-,37-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002713
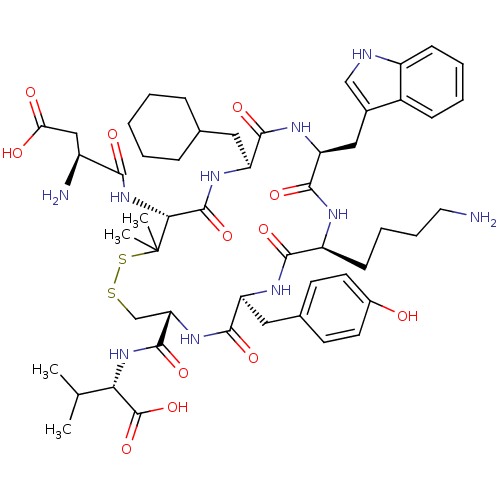
(CHEMBL425467)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C52H74N10O12S2/c1-28(2)42(51(73)74)61-49(71)40-27-75-76-52(3,4)43(62-44(66)34(54)25-41(64)65)50(72)59-38(22-29-12-6-5-7-13-29)46(68)58-39(24-31-26-55-35-15-9-8-14-33(31)35)48(70)56-36(16-10-11-21-53)45(67)57-37(47(69)60-40)23-30-17-19-32(63)20-18-30/h8-9,14-15,17-20,26,28-29,34,36-40,42-43,55,63H,5-7,10-13,16,21-25,27,53-54H2,1-4H3,(H,56,70)(H,57,67)(H,58,68)(H,59,72)(H,60,69)(H,61,71)(H,62,66)(H,64,65)(H,73,74)/t34-,36-,37-,38-,39-,40-,42-,43+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50002698
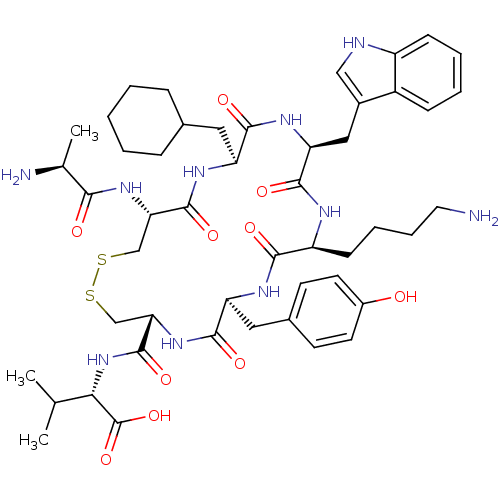
(CHEMBL437110)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C49H70N10O10S2/c1-27(2)41(49(68)69)59-48(67)40-26-71-70-25-39(57-42(61)28(3)51)47(66)55-36(21-29-11-5-4-6-12-29)44(63)56-38(23-31-24-52-34-14-8-7-13-33(31)34)46(65)53-35(15-9-10-20-50)43(62)54-37(45(64)58-40)22-30-16-18-32(60)19-17-30/h7-8,13-14,16-19,24,27-29,35-41,52,60H,4-6,9-12,15,20-23,25-26,50-51H2,1-3H3,(H,53,65)(H,54,62)(H,55,66)(H,56,63)(H,57,61)(H,58,64)(H,59,67)(H,68,69)/t28-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]human urotensin-2 from human GPR14 transfected in CHO cells |
J Med Chem 49: 7234-8 (2006)
Checked by Author
Article DOI: 10.1021/jm0602110
BindingDB Entry DOI: 10.7270/Q29G5P1V |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM85080

(CH-275 | CH275 | L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-...)Show SMILES CC(C)NCc1ccc(C[C@H]2NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)C(Cc3ccccc3)NC(=O)C(Cc3ccccc3)NC(=O)C(CCCCN)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC(=O)C(NC2=O)C(C)O)C(C)O)C(O)=O)cc1 Show InChI InChI=1S/C74H96N14O15S2/c1-42(2)77-37-49-29-27-48(28-30-49)35-57-69(97)87-62(43(3)90)72(100)84-58(34-47-22-12-7-13-23-47)70(98)88-63(44(4)91)73(101)85-60(39-89)71(99)86-61(74(102)103)41-105-104-40-52(76)64(92)79-54(26-16-17-31-75)65(93)80-55(32-45-18-8-5-9-19-45)66(94)81-56(33-46-20-10-6-11-21-46)67(95)83-59(68(96)82-57)36-50-38-78-53-25-15-14-24-51(50)53/h5-15,18-25,27-30,38,42-44,52,54-63,77-78,89-91H,16-17,26,31-37,39-41,75-76H2,1-4H3,(H,79,92)(H,80,93)(H,81,94)(H,82,96)(H,83,95)(H,84,100)(H,85,101)(H,86,99)(H,87,97)(H,88,98)(H,102,103)/t43?,44?,52-,54?,55?,56?,57-,58-,59+,60-,61-,62?,63+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM84629

(BIM 23056)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C71H81N11O9/c1-44(2)63(71(91)81-61(39-47-23-10-5-11-24-47)67(87)77-58(64(74)84)41-50-27-18-26-49-25-12-13-28-53(49)50)82-66(86)57(31-16-17-36-72)76-70(90)62(42-51-43-75-56-30-15-14-29-54(51)56)80-69(89)60(40-48-32-34-52(83)35-33-48)79-68(88)59(38-46-21-8-4-9-22-46)78-65(85)55(73)37-45-19-6-3-7-20-45/h3-15,18-30,32-35,43-44,55,57-63,75,83H,16-17,31,36-42,72-73H2,1-2H3,(H2,74,84)(H,76,90)(H,77,87)(H,78,85)(H,79,88)(H,80,89)(H,81,91)(H,82,86)/t55-,57+,58-,59+,60+,61+,62-,63+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM U159
Curated by PDSP Ki Database
| |
J Neurochem 68: 2263-72 (1997)
Article DOI: 10.1046/j.1471-4159.1997.68062263.x
BindingDB Entry DOI: 10.7270/Q2Z899X9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data