 Found 100 hits with Last Name = 'villinger' and Initial = 'a'
Found 100 hits with Last Name = 'villinger' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50412149
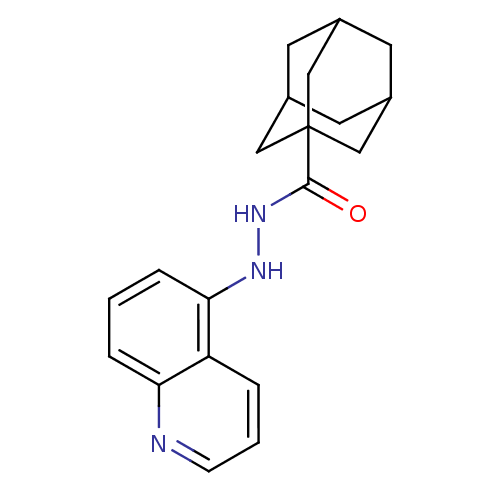
(CHEMBL497967)Show SMILES O=C(NNc1cccc2ncccc12)C12CC3CC(CC(C3)C1)C2 |TLB:1:14:17:21.20.19,THB:15:16:19:23.14.22,15:14:17.16.21:19,22:14:17:21.20.19,22:20:17:23.15.14| Show InChI InChI=1S/C20H23N3O/c24-19(20-10-13-7-14(11-20)9-15(8-13)12-20)23-22-18-5-1-4-17-16(18)3-2-6-21-17/h1-6,13-15,22H,7-12H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50386566
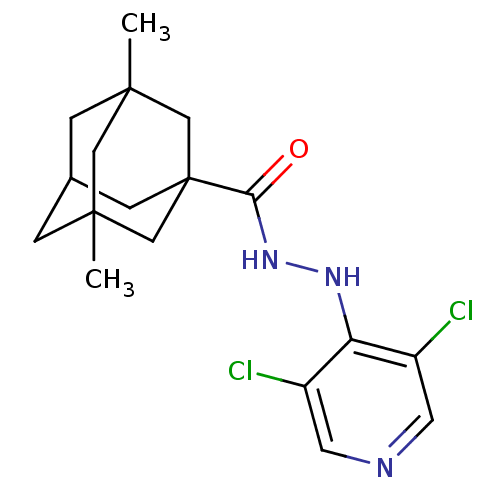
(CHEMBL2048438)Show SMILES CC12CC3CC(C)(C1)CC(C3)(C2)C(=O)NNc1c(Cl)cncc1Cl |TLB:12:9:4:2.7.1,7:1:4.5.8:10,0:1:4:8.9.10,THB:7:5:10:2.1.11,6:5:10:2.1.11,11:1:4:8.9.10,11:9:4:2.7.1| Show InChI InChI=1S/C18H23Cl2N3O/c1-16-3-11-4-17(2,8-16)10-18(5-11,9-16)15(24)23-22-14-12(19)6-21-7-13(14)20/h6-7,11H,3-5,8-10H2,1-2H3,(H,21,22)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598327
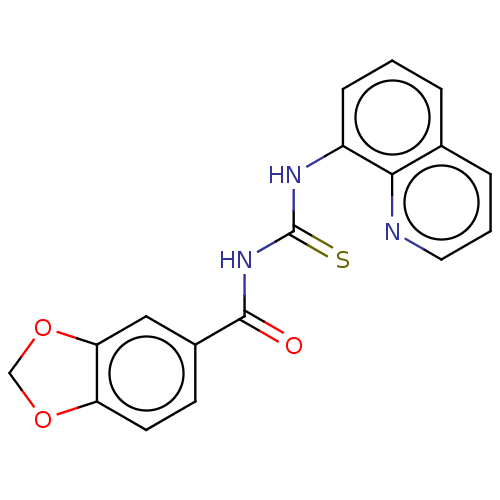
(CHEMBL5178892) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598327

(CHEMBL5178892) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598318
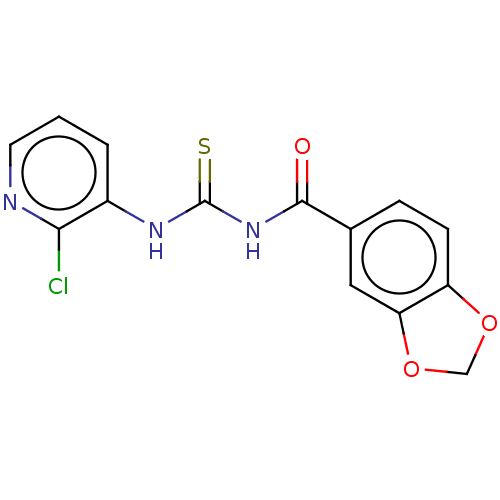
(CHEMBL5205239) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598318

(CHEMBL5205239) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598326
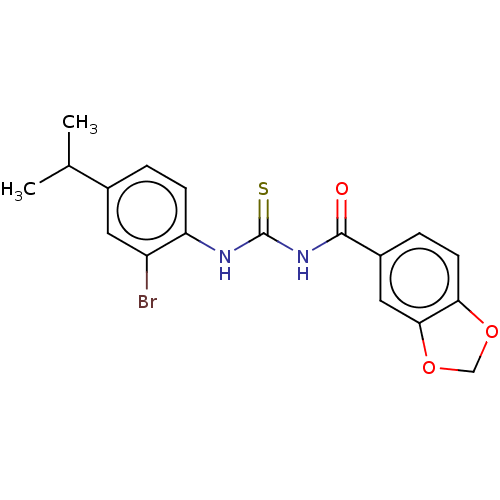
(CHEMBL5206892)Show SMILES CC(C)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)c(Br)c1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598320
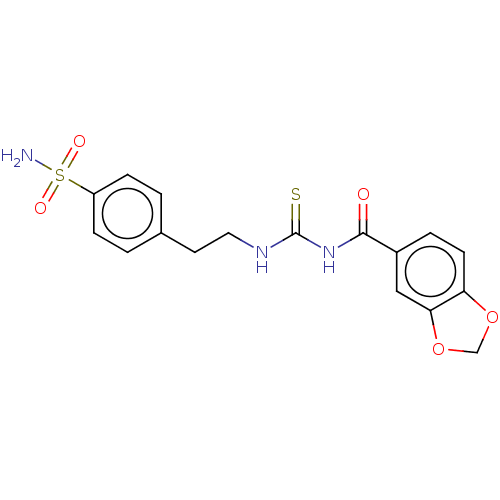
(CHEMBL5174305)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598320

(CHEMBL5174305)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598322
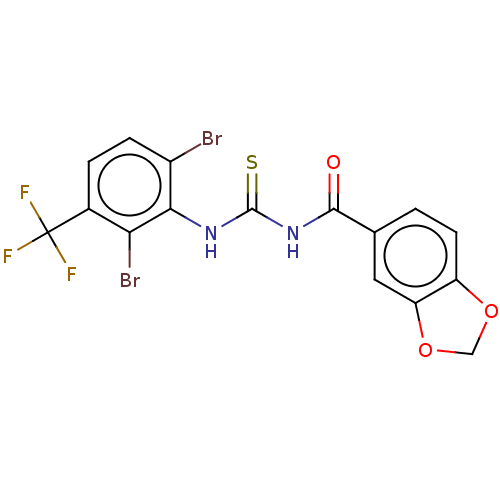
(CHEMBL5200114)Show SMILES FC(F)(F)c1ccc(Br)c(NC(=S)NC(=O)c2ccc3OCOc3c2)c1Br | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598319
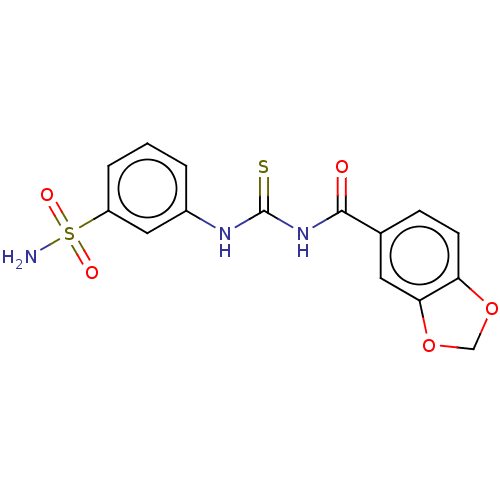
(CHEMBL5194724)Show SMILES NS(=O)(=O)c1cccc(NC(=S)NC(=O)c2ccc3OCOc3c2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598319

(CHEMBL5194724)Show SMILES NS(=O)(=O)c1cccc(NC(=S)NC(=O)c2ccc3OCOc3c2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598316
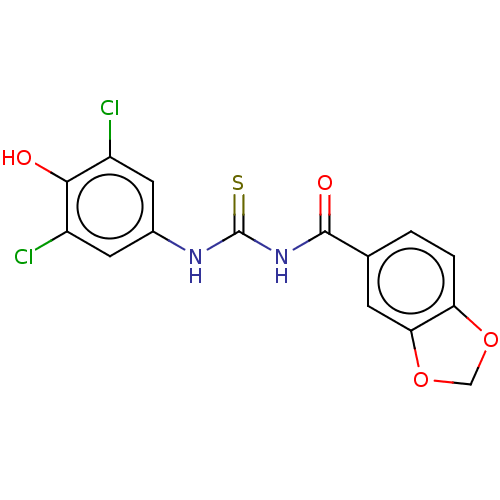
(CHEMBL5184613) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 634 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 902 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50506159
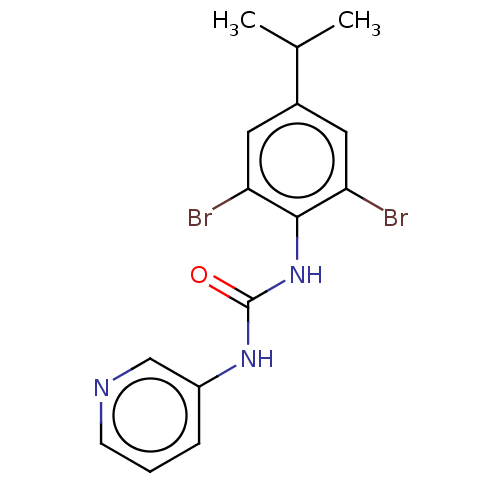
(CHEMBL1532400)Show InChI InChI=1S/C15H15Br2N3O/c1-9(2)10-6-12(16)14(13(17)7-10)20-15(21)19-11-4-3-5-18-8-11/h3-9H,1-2H3,(H2,19,20,21) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 904 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598327

(CHEMBL5178892) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 908 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598318

(CHEMBL5205239) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 935 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598320

(CHEMBL5174305)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234715
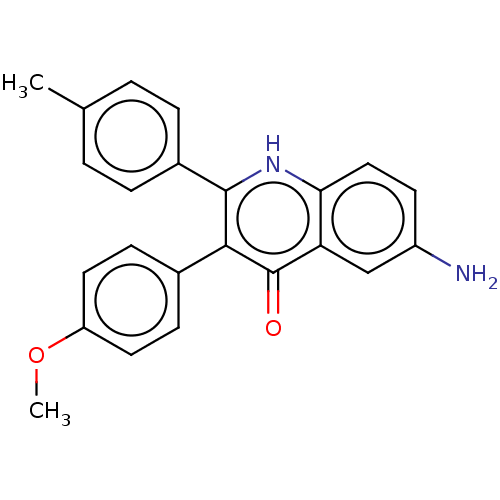
(CHEMBL4069497)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccc(N)cc2c1=O)-c1ccc(C)cc1 Show InChI InChI=1S/C23H20N2O2/c1-14-3-5-16(6-4-14)22-21(15-7-10-18(27-2)11-8-15)23(26)19-13-17(24)9-12-20(19)25-22/h3-13H,24H2,1-2H3,(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598321
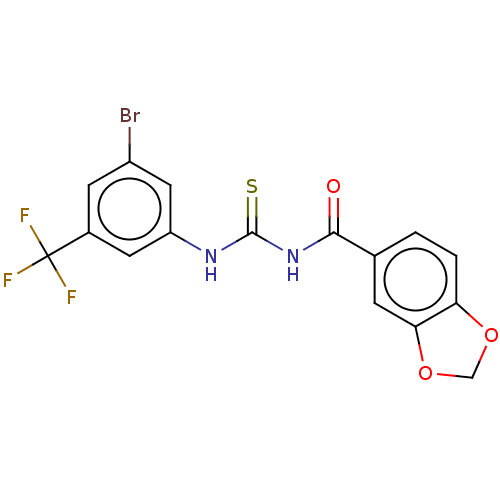
(CHEMBL5172890)Show SMILES FC(F)(F)c1cc(Br)cc(NC(=S)NC(=O)c2ccc3OCOc3c2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234702

(CHEMBL4066927)Show SMILES CC(c1ccccc1)n1c(-c2ccc(C)cc2)c(Br)c(=O)c2cc(ccc12)[N+]([O-])=O Show InChI InChI=1S/C24H19BrN2O3/c1-15-8-10-18(11-9-15)23-22(25)24(28)20-14-19(27(29)30)12-13-21(20)26(23)16(2)17-6-4-3-5-7-17/h3-14,16H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234719

(CHEMBL4062180)Show SMILES CCCCCc1cc(=O)c2cc(ccc2n1Cc1ccc(OC)cc1)[N+]([O-])=O Show InChI InChI=1S/C22H24N2O4/c1-3-4-5-6-17-14-22(25)20-13-18(24(26)27)9-12-21(20)23(17)15-16-7-10-19(28-2)11-8-16/h7-14H,3-6,15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598313

(CHEMBL5191762) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234719

(CHEMBL4062180)Show SMILES CCCCCc1cc(=O)c2cc(ccc2n1Cc1ccc(OC)cc1)[N+]([O-])=O Show InChI InChI=1S/C22H24N2O4/c1-3-4-5-6-17-14-22(25)20-13-18(24(26)27)9-12-21(20)23(17)15-16-7-10-19(28-2)11-8-16/h7-14H,3-6,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598316

(CHEMBL5184613) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234703
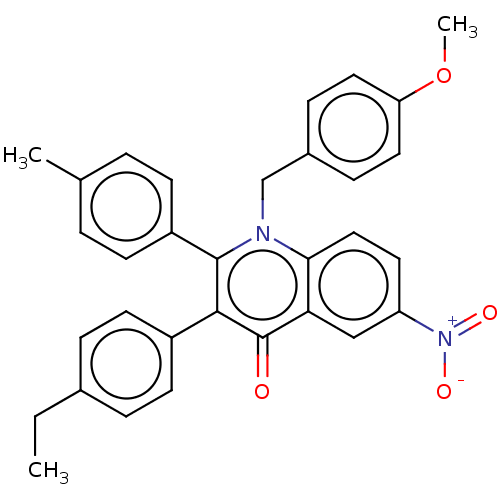
(CHEMBL4104578)Show SMILES CCc1ccc(cc1)-c1c(-c2ccc(C)cc2)n(Cc2ccc(OC)cc2)c2ccc(cc2c1=O)[N+]([O-])=O Show InChI InChI=1S/C32H28N2O4/c1-4-22-7-13-24(14-8-22)30-31(25-11-5-21(2)6-12-25)33(20-23-9-16-27(38-3)17-10-23)29-18-15-26(34(36)37)19-28(29)32(30)35/h5-19H,4,20H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234716
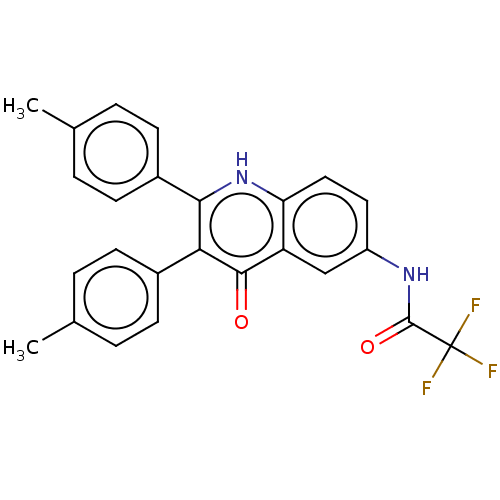
(CHEMBL4104441)Show SMILES Cc1ccc(cc1)-c1[nH]c2ccc(NC(=O)C(F)(F)F)cc2c(=O)c1-c1ccc(C)cc1 Show InChI InChI=1S/C25H19F3N2O2/c1-14-3-7-16(8-4-14)21-22(17-9-5-15(2)6-10-17)30-20-12-11-18(13-19(20)23(21)31)29-24(32)25(26,27)28/h3-13H,1-2H3,(H,29,32)(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50540409
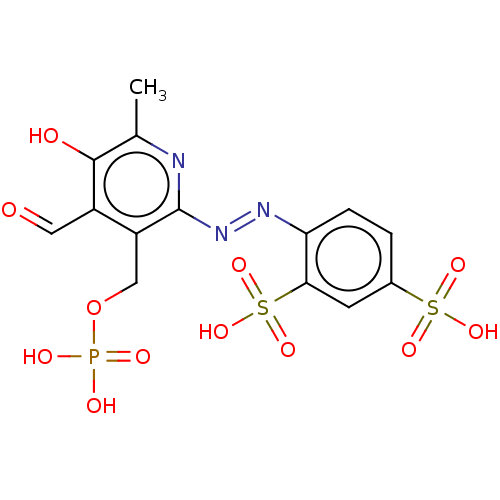
(CHEBI:34941 | CHEMBL69234)Show SMILES Cc1nc(\N=N\c2ccc(cc2S(O)(=O)=O)S(O)(=O)=O)c(COP(O)(O)=O)c(C=O)c1O Show InChI InChI=1S/C14H14N3O12PS2/c1-7-13(19)9(5-18)10(6-29-30(20,21)22)14(15-7)17-16-11-3-2-8(31(23,24)25)4-12(11)32(26,27)28/h2-5,19H,6H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)/b17-16+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234717
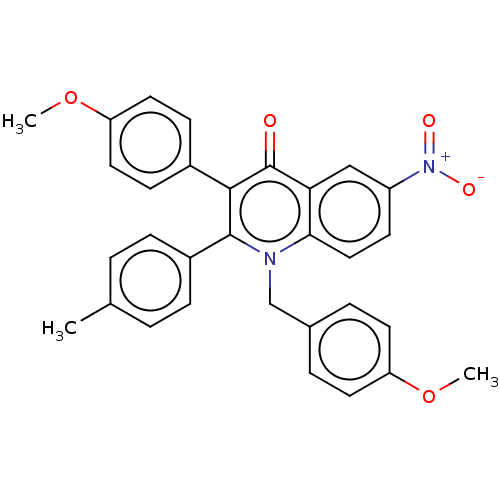
(CHEMBL4076852)Show SMILES COc1ccc(Cn2c(-c3ccc(C)cc3)c(-c3ccc(OC)cc3)c(=O)c3cc(ccc23)[N+]([O-])=O)cc1 Show InChI InChI=1S/C31H26N2O5/c1-20-4-8-23(9-5-20)30-29(22-10-15-26(38-3)16-11-22)31(34)27-18-24(33(35)36)12-17-28(27)32(30)19-21-6-13-25(37-2)14-7-21/h4-18H,19H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50598314
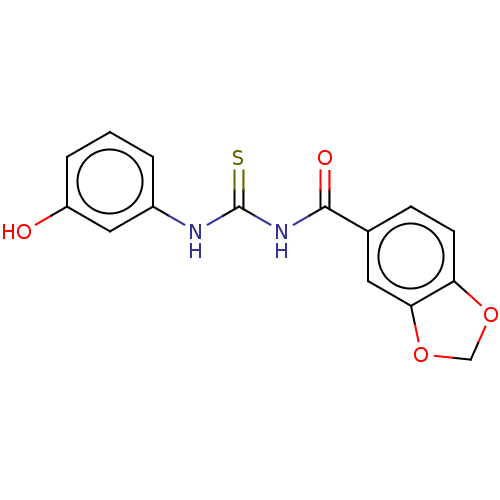
(CHEMBL5190137) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598313

(CHEMBL5191762) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598319

(CHEMBL5194724)Show SMILES NS(=O)(=O)c1cccc(NC(=S)NC(=O)c2ccc3OCOc3c2)c1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234721
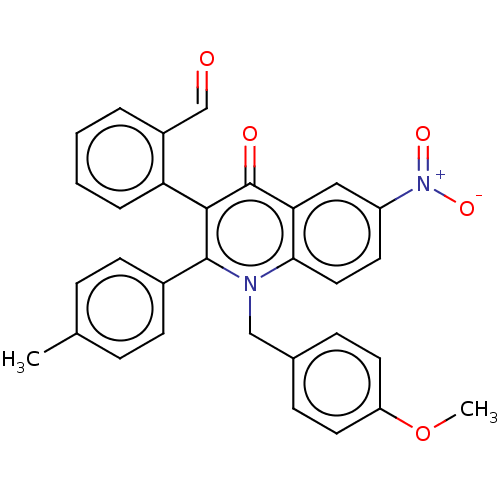
(CHEMBL4081390)Show SMILES COc1ccc(Cn2c(-c3ccc(C)cc3)c(-c3ccccc3C=O)c(=O)c3cc(ccc23)[N+]([O-])=O)cc1 |(41.76,-40.13,;43.1,-40.89,;44.43,-40.12,;44.42,-38.57,;45.75,-37.8,;47.09,-38.57,;48.42,-37.8,;48.41,-36.26,;49.76,-35.48,;51.09,-36.25,;51.09,-37.79,;52.42,-38.55,;53.76,-37.78,;55.09,-38.54,;53.75,-36.23,;52.41,-35.47,;49.75,-33.93,;51.09,-33.16,;52.42,-33.93,;53.75,-33.15,;53.75,-31.61,;52.4,-30.85,;51.08,-31.62,;49.74,-30.86,;49.73,-29.32,;48.4,-33.15,;48.39,-31.61,;47.06,-33.94,;45.73,-33.17,;44.4,-33.94,;44.4,-35.49,;45.73,-36.26,;47.07,-35.49,;43.07,-33.17,;43.07,-31.64,;41.73,-33.95,;47.1,-40.11,;45.77,-40.88,)| Show InChI InChI=1S/C31H24N2O5/c1-20-7-11-22(12-8-20)30-29(26-6-4-3-5-23(26)19-34)31(35)27-17-24(33(36)37)13-16-28(27)32(30)18-21-9-14-25(38-2)15-10-21/h3-17,19H,18H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234701

(CHEMBL4094414)Show SMILES COc1ccc(Cn2c(-c3ccc(C)cc3)c(Br)c(=O)c3cc(ccc23)[N+]([O-])=O)cc1 Show InChI InChI=1S/C24H19BrN2O4/c1-15-3-7-17(8-4-15)23-22(25)24(28)20-13-18(27(29)30)9-12-21(20)26(23)14-16-5-10-19(31-2)11-6-16/h3-13H,14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234702

(CHEMBL4066927)Show SMILES CC(c1ccccc1)n1c(-c2ccc(C)cc2)c(Br)c(=O)c2cc(ccc12)[N+]([O-])=O Show InChI InChI=1S/C24H19BrN2O3/c1-15-8-10-18(11-9-15)23-22(25)24(28)20-14-19(27(29)30)12-13-21(20)26(23)16(2)17-6-4-3-5-7-17/h3-14,16H,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234710

(CHEMBL4084870)Show SMILES Cc1ccc(cc1)-c1cc(=O)c2cc(ccc2[nH]1)[N+]([O-])=O Show InChI InChI=1S/C16H12N2O3/c1-10-2-4-11(5-3-10)15-9-16(19)13-8-12(18(20)21)6-7-14(13)17-15/h2-9H,1H3,(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234713

(CHEMBL4077772)Show SMILES Cc1ccc(cc1)-c1[nH]c2ccc(cc2c(=O)c1-c1ccc(cc1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C23H15F3N2O3/c1-13-2-4-15(5-3-13)21-20(14-6-8-16(9-7-14)23(24,25)26)22(29)18-12-17(28(30)31)10-11-19(18)27-21/h2-12H,1H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234718
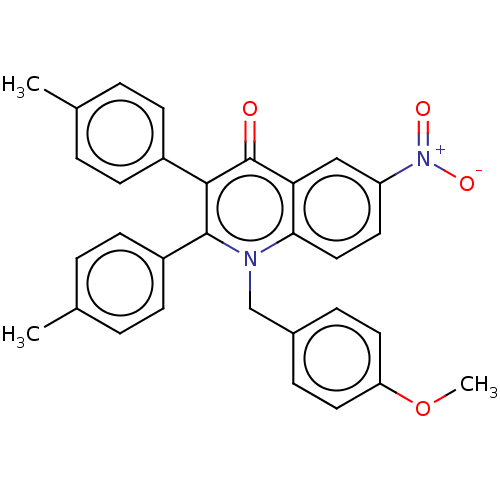
(CHEMBL4097906)Show SMILES COc1ccc(Cn2c(-c3ccc(C)cc3)c(-c3ccc(C)cc3)c(=O)c3cc(ccc23)[N+]([O-])=O)cc1 Show InChI InChI=1S/C31H26N2O4/c1-20-4-10-23(11-5-20)29-30(24-12-6-21(2)7-13-24)32(19-22-8-15-26(37-3)16-9-22)28-17-14-25(33(35)36)18-27(28)31(29)34/h4-18H,19H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234712
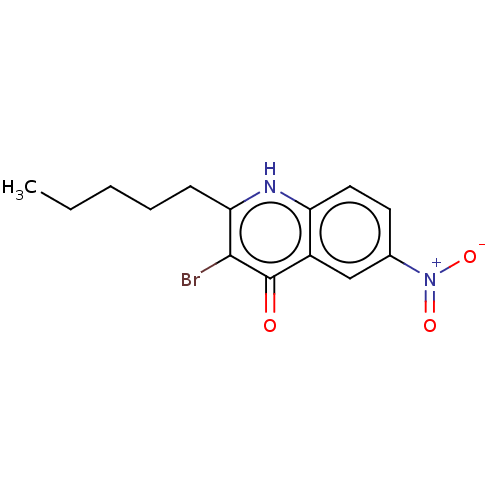
(CHEMBL4088077)Show InChI InChI=1S/C14H15BrN2O3/c1-2-3-4-5-12-13(15)14(18)10-8-9(17(19)20)6-7-11(10)16-12/h6-8H,2-5H2,1H3,(H,16,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50598322

(CHEMBL5200114)Show SMILES FC(F)(F)c1ccc(Br)c(NC(=S)NC(=O)c2ccc3OCOc3c2)c1Br | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598323
(CHEMBL5173949) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234701

(CHEMBL4094414)Show SMILES COc1ccc(Cn2c(-c3ccc(C)cc3)c(Br)c(=O)c3cc(ccc23)[N+]([O-])=O)cc1 Show InChI InChI=1S/C24H19BrN2O4/c1-15-3-7-17(8-4-15)23-22(25)24(28)20-13-18(27(29)30)9-12-21(20)26(23)14-16-5-10-19(31-2)11-6-16/h3-13H,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Intestinal-type alkaline phosphatase
(Bos taurus (Cattle)) | BDBM50234713

(CHEMBL4077772)Show SMILES Cc1ccc(cc1)-c1[nH]c2ccc(cc2c(=O)c1-c1ccc(cc1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C23H15F3N2O3/c1-13-2-4-15(5-3-13)21-20(14-6-8-16(9-7-14)23(24,25)26)22(29)18-12-17(28(30)31)10-11-19(18)27-21/h2-12H,1H3,(H,27,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of calf intestinal alkaline phosphatase using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured a... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Bos taurus (Cattle)) | BDBM50234709

(CHEMBL4090195)Show SMILES COc1ccc(Cn2c(-c3ccc(C)cc3)c(c(=O)c3cc(ccc23)[N+]([O-])=O)C(F)(F)F)cc1 Show InChI InChI=1S/C25H19F3N2O4/c1-15-3-7-17(8-4-15)23-22(25(26,27)28)24(31)20-13-18(30(32)33)9-12-21(20)29(23)14-16-5-10-19(34-2)11-6-16/h3-13H,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of bovine TNAP using CDP-star as substrate preincubated for 5 to 7 mins followed by substrate addition measured after 15 mins by spectroph... |
Eur J Med Chem 126: 408-420 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.036
BindingDB Entry DOI: 10.7270/Q2DZ0BKR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data