 Found 63 hits with Last Name = 'vinsova' and Initial = 'j'
Found 63 hits with Last Name = 'vinsova' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115
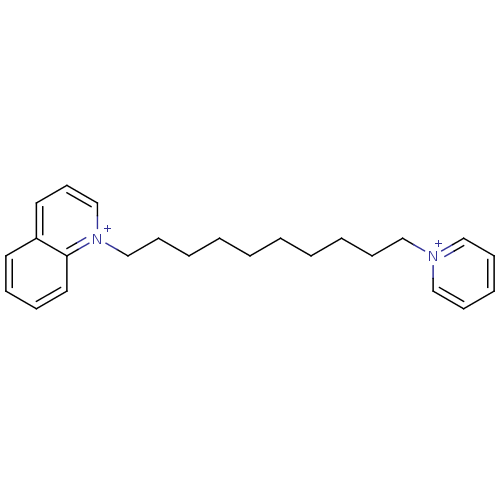
(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115

(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262
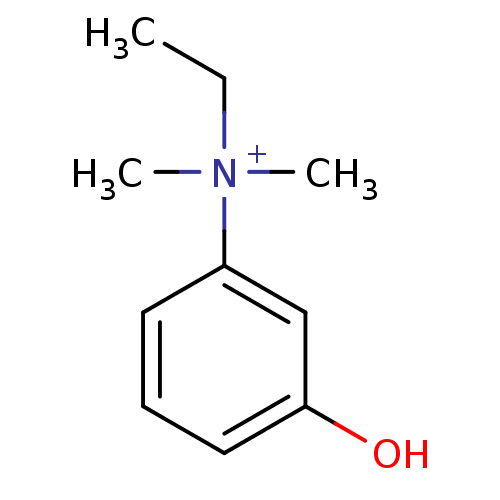
(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115

(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005116

(CHEMBL3093803)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccccc1)CCCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C25H34N2.2BrH/c1(2-4-6-11-19-26-20-12-8-13-21-26)3-5-7-14-22-27-23-15-17-24-16-9-10-18-25(24)27;;/h8-10,12-13,15-18,20-21,23H,1-7,11,14,19,22H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005181

(CHEMBL3093797)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccc2ccccc2c1)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-17-25-18-12-7-13-19-25)2-4-6-11-20-26-21-16-23-14-8-9-15-24(23)22-26;;/h7-9,12-16,18-19,21-22H,1-6,10-11,17,20H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005180

(CHEMBL3093798)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccccc1)CCCCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C25H34N2.2BrH/c1(2-4-6-11-18-26-19-13-8-14-20-26)3-5-7-12-21-27-22-17-24-15-9-10-16-25(24)23-27;;/h8-10,13-17,19-20,22-23H,1-7,11-12,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005182

(CHEMBL3093796)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccccc1)CCCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C23H30N2.2BrH/c1(2-4-9-16-24-17-11-6-12-18-24)3-5-10-19-25-20-15-22-13-7-8-14-23(22)21-25;;/h6-8,11-15,17-18,20-21H,1-5,9-10,16,19H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005119
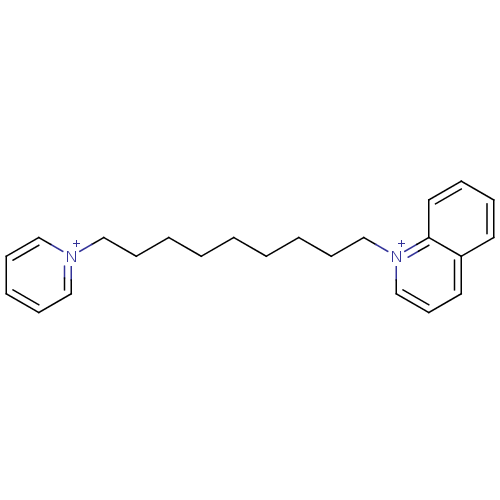
(CHEMBL3093801)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccccc1)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C23H30N2.2BrH/c1(2-4-9-17-24-18-10-6-11-19-24)3-5-12-20-25-21-13-15-22-14-7-8-16-23(22)25;;/h6-8,10-11,13-16,18-19,21H,1-5,9,12,17,20H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005184

(CHEMBL3093795)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccc2ccccc2c1)CCC[n+]1ccccc1 Show InChI InChI=1S/C22H28N2.2BrH/c1(3-8-15-23-16-10-5-11-17-23)2-4-9-18-24-19-14-21-12-6-7-13-22(21)20-24;;/h5-7,10-14,16-17,19-20H,1-4,8-9,15,18H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005171

(CHEMBL3093800)Show SMILES [Br-].[Br-].C(CCCC[n+]1cccc2ccccc12)CCC[n+]1ccccc1 Show InChI InChI=1S/C22H28N2.2BrH/c1(3-8-16-23-17-9-5-10-18-23)2-4-11-19-24-20-12-14-21-13-6-7-15-22(21)24;;/h5-7,9-10,12-15,17-18,20H,1-4,8,11,16,19H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005182

(CHEMBL3093796)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccccc1)CCCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C23H30N2.2BrH/c1(2-4-9-16-24-17-11-6-12-18-24)3-5-10-19-25-20-15-22-13-7-8-14-23(22)21-25;;/h6-8,11-15,17-18,20-21H,1-5,9-10,16,19H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005181

(CHEMBL3093797)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccc2ccccc2c1)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-17-25-18-12-7-13-19-25)2-4-6-11-20-26-21-16-23-14-8-9-15-24(23)22-26;;/h7-9,12-16,18-19,21-22H,1-6,10-11,17,20H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005179

(CHEMBL3093799)Show SMILES [Br-].[Br-].C(CCC[n+]1ccccc1)CCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C21H26N2.2BrH/c1(2-7-15-22-16-8-4-9-17-22)3-10-18-23-19-11-13-20-12-5-6-14-21(20)23;;/h4-6,8-9,11-14,16-17,19H,1-3,7,10,15,18H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005180

(CHEMBL3093798)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccccc1)CCCCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C25H34N2.2BrH/c1(2-4-6-11-18-26-19-13-8-14-20-26)3-5-7-12-21-27-22-17-24-15-9-10-16-25(24)23-27;;/h8-10,13-17,19-20,22-23H,1-7,11-12,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005185

(CHEMBL3093794)Show SMILES [Br-].[Br-].C(CCC[n+]1ccccc1)CCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C21H26N2.2BrH/c1(2-7-14-22-15-9-4-10-16-22)3-8-17-23-18-13-20-11-5-6-12-21(20)19-23;;/h4-6,9-13,15-16,18-19H,1-3,7-8,14,17H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005116

(CHEMBL3093803)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccccc1)CCCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C25H34N2.2BrH/c1(2-4-6-11-19-26-20-12-8-13-21-26)3-5-7-14-22-27-23-15-17-24-16-9-10-18-25(24)27;;/h8-10,12-13,15-18,20-21,23H,1-7,11,14,19,22H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005185

(CHEMBL3093794)Show SMILES [Br-].[Br-].C(CCC[n+]1ccccc1)CCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C21H26N2.2BrH/c1(2-7-14-22-15-9-4-10-16-22)3-8-17-23-18-13-20-11-5-6-12-21(20)19-23;;/h4-6,9-13,15-16,18-19H,1-3,7-8,14,17H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005119

(CHEMBL3093801)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccccc1)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C23H30N2.2BrH/c1(2-4-9-17-24-18-10-6-11-19-24)3-5-12-20-25-21-13-15-22-14-7-8-16-23(22)25;;/h6-8,10-11,13-16,18-19,21H,1-5,9,12,17,20H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50487027

(CHEBI:116509 | DCMU | Diuron)Show InChI InChI=1S/C9H10Cl2N2O/c1-13(2)9(14)12-6-3-4-7(10)8(11)5-6/h3-5H,1-2H3,(H,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005171

(CHEMBL3093800)Show SMILES [Br-].[Br-].C(CCCC[n+]1cccc2ccccc12)CCC[n+]1ccccc1 Show InChI InChI=1S/C22H28N2.2BrH/c1(3-8-16-23-17-9-5-10-18-23)2-4-11-19-24-20-12-14-21-13-6-7-15-22(21)24;;/h5-7,9-10,12-15,17-18,20H,1-4,8,11,16,19H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005179

(CHEMBL3093799)Show SMILES [Br-].[Br-].C(CCC[n+]1ccccc1)CCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C21H26N2.2BrH/c1(2-7-15-22-16-8-4-9-17-22)3-10-18-23-19-11-13-20-12-5-6-14-21(20)23;;/h4-6,8-9,11-14,16-17,19H,1-3,7,10,15,18H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005115

(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488542

(CHEMBL602140)Show SMILES CCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C21H24Cl2N2O3/c1-2-3-4-5-6-12-24-21(27)28-19-11-10-16(23)14-18(19)20(26)25-17-9-7-8-15(22)13-17/h7-11,13-14H,2-6,12H2,1H3,(H,24,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262

(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488560
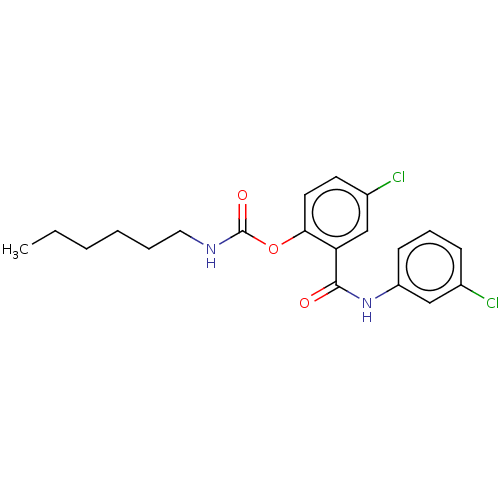
(CHEMBL611390)Show SMILES CCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C20H22Cl2N2O3/c1-2-3-4-5-11-23-20(26)27-18-10-9-15(22)13-17(18)19(25)24-16-8-6-7-14(21)12-16/h6-10,12-13H,2-5,11H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488559
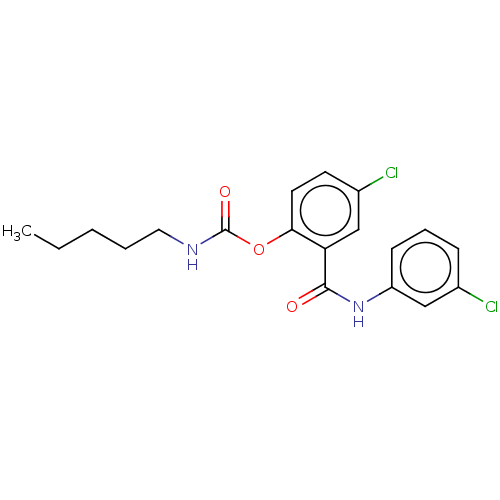
(CHEMBL600467)Show InChI InChI=1S/C19H20Cl2N2O3/c1-2-3-4-10-22-19(25)26-17-9-8-14(21)12-16(17)18(24)23-15-7-5-6-13(20)11-15/h5-9,11-12H,2-4,10H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488562
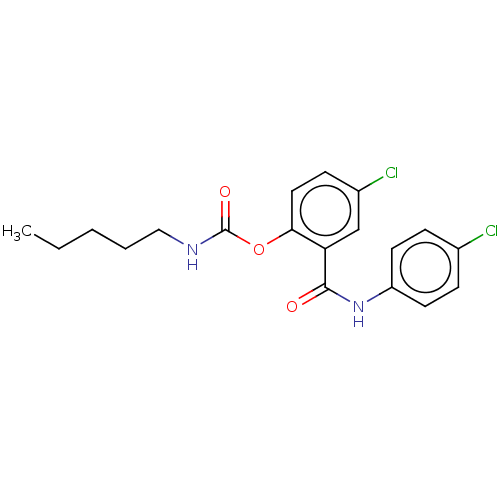
(CHEMBL601636)Show InChI InChI=1S/C19H20Cl2N2O3/c1-2-3-4-11-22-19(25)26-17-10-7-14(21)12-16(17)18(24)23-15-8-5-13(20)6-9-15/h5-10,12H,2-4,11H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488561

(CHEMBL601851)Show SMILES CCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C20H22Cl2N2O3/c1-2-3-4-5-12-23-20(26)27-18-11-8-15(22)13-17(18)19(25)24-16-9-6-14(21)7-10-16/h6-11,13H,2-5,12H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488541
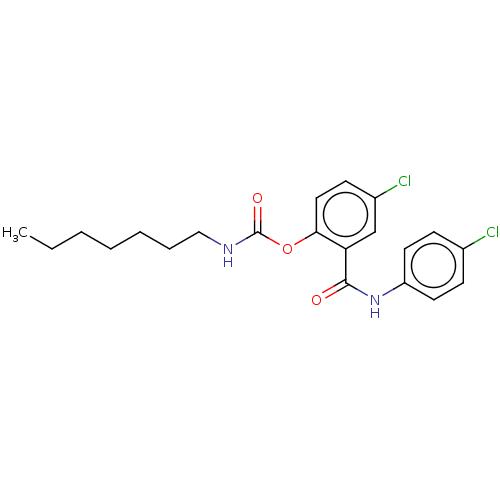
(CHEMBL602056)Show SMILES CCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C21H24Cl2N2O3/c1-2-3-4-5-6-13-24-21(27)28-19-12-9-16(23)14-18(19)20(26)25-17-10-7-15(22)8-11-17/h7-12,14H,2-6,13H2,1H3,(H,24,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488549
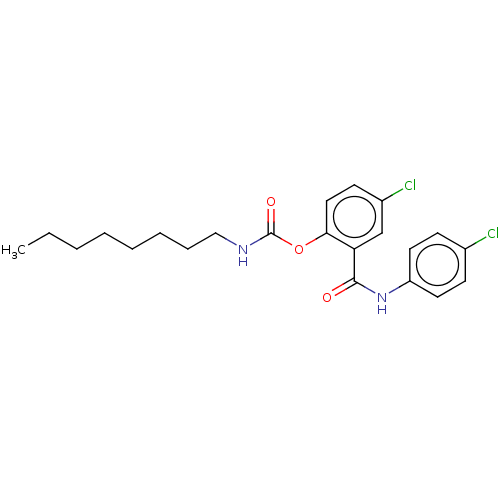
(CHEMBL604133)Show SMILES CCCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C22H26Cl2N2O3/c1-2-3-4-5-6-7-14-25-22(28)29-20-13-10-17(24)15-19(20)21(27)26-18-11-8-16(23)9-12-18/h8-13,15H,2-7,14H2,1H3,(H,25,28)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50005184

(CHEMBL3093795)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccc2ccccc2c1)CCC[n+]1ccccc1 Show InChI InChI=1S/C22H28N2.2BrH/c1(3-8-15-23-16-10-5-11-17-23)2-4-9-18-24-19-14-21-12-6-7-13-22(21)20-24;;/h5-7,10-14,16-17,19-20H,1-4,8-9,15,18H2;2*1H/q+2;;/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488548
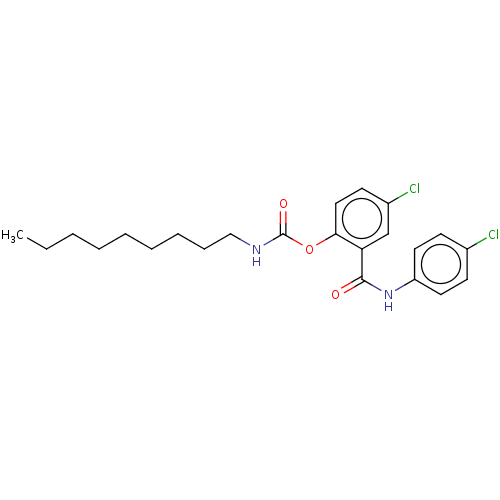
(CHEMBL601853)Show SMILES CCCCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C23H28Cl2N2O3/c1-2-3-4-5-6-7-8-15-26-23(29)30-21-14-11-18(25)16-20(21)22(28)27-19-12-9-17(24)10-13-19/h9-14,16H,2-8,15H2,1H3,(H,26,29)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488547

(CHEMBL602055)Show SMILES CCCCCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C24H30Cl2N2O3/c1-2-3-4-5-6-7-8-9-16-27-24(30)31-22-15-12-19(26)17-21(22)23(29)28-20-13-10-18(25)11-14-20/h10-15,17H,2-9,16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488550

(CHEMBL2287510)Show InChI InChI=1S/C17H16Cl2N2O3/c1-2-9-20-17(23)24-15-8-5-12(19)10-14(15)16(22)21-13-6-3-11(18)4-7-13/h3-8,10H,2,9H2,1H3,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488551
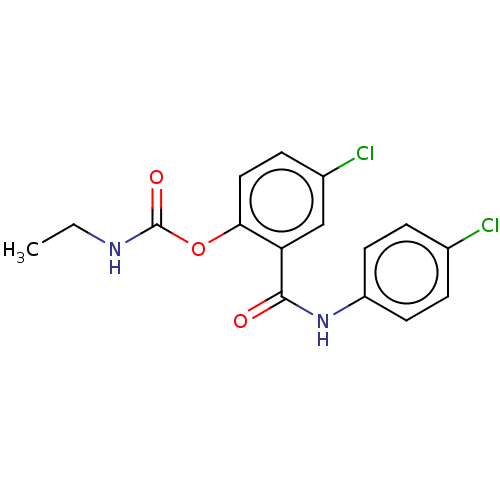
(CHEMBL601242)Show InChI InChI=1S/C16H14Cl2N2O3/c1-2-19-16(22)23-14-8-5-11(18)9-13(14)15(21)20-12-6-3-10(17)4-7-12/h3-9H,2H2,1H3,(H,19,22)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488553
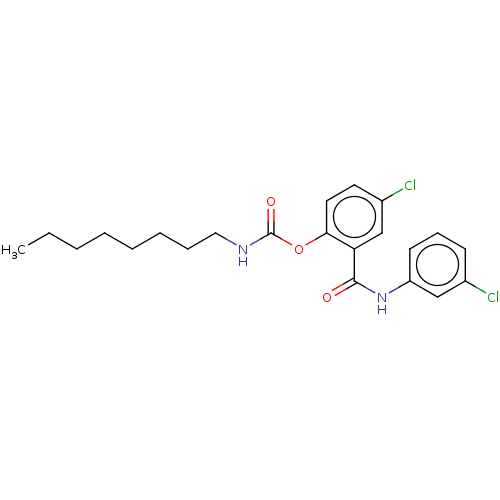
(CHEMBL611667)Show SMILES CCCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C22H26Cl2N2O3/c1-2-3-4-5-6-7-13-25-22(28)29-20-12-11-17(24)15-19(20)21(27)26-18-10-8-9-16(23)14-18/h8-12,14-15H,2-7,13H2,1H3,(H,25,28)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488557

(CHEMBL598736)Show SMILES CCCCCCCCCCCNC(=O)Oc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C25H32Cl2N2O3/c1-2-3-4-5-6-7-8-9-10-17-28-25(31)32-23-16-13-20(27)18-22(23)24(30)29-21-14-11-19(26)12-15-21/h11-16,18H,2-10,17H2,1H3,(H,28,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |
Photosystem II protein D1
(Spinacia oleracea) | BDBM50488564
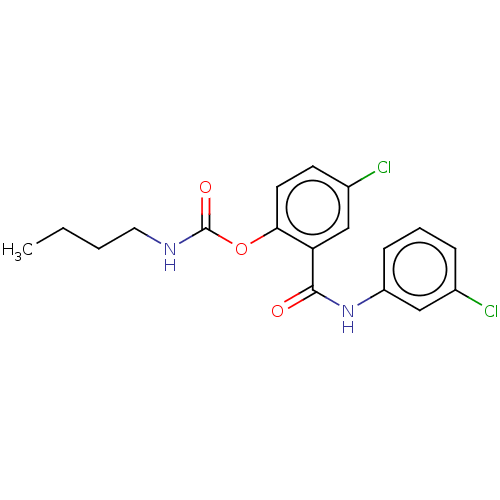
(CHEMBL599560)Show InChI InChI=1S/C18H18Cl2N2O3/c1-2-3-9-21-18(24)25-16-8-7-13(20)11-15(16)17(23)22-14-6-4-5-12(19)10-14/h4-8,10-11H,2-3,9H2,1H3,(H,21,24)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pardubice
Curated by ChEMBL
| Assay Description
Inhibition of photosystem II in Spinacia oleracea (spinach) chloroplasts assessed as reduction of photosynthetic electron transport |
Bioorg Med Chem Lett 21: 4564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.118
BindingDB Entry DOI: 10.7270/Q26D5WWX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data