

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254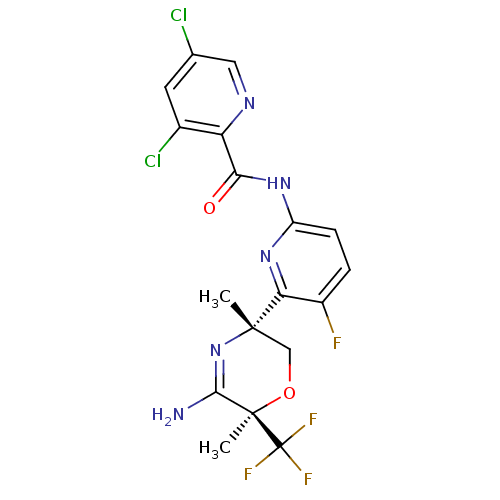 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate with BACE-cleavable sequence | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US8637508 (2014) BindingDB Entry DOI: 10.7270/Q2JD4VFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10683287 (2020) BindingDB Entry DOI: 10.7270/Q2DZ0CCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50587074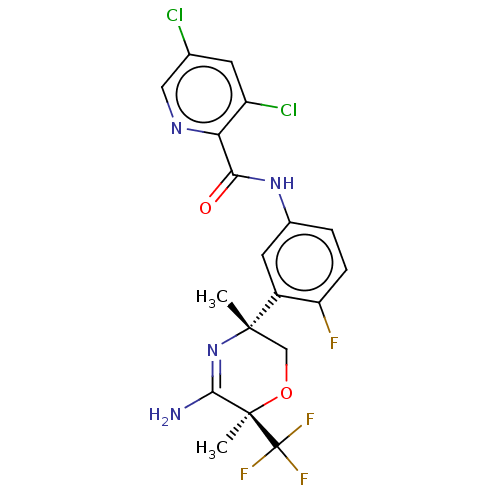 (CHEMBL5085959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM116236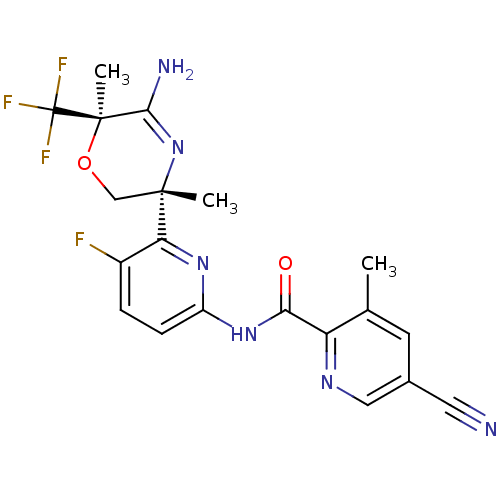 (US10035794, Example 11 | US10683287, Example 11 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well i... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50506051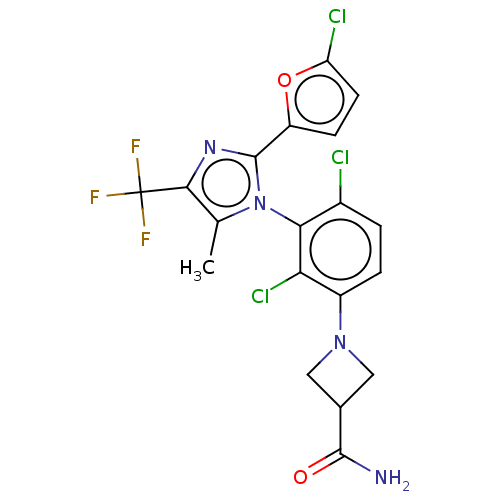 (CHEMBL4587988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Discovery Chemistry Curated by ChEMBL | Assay Description Displacement of RIP140 cofactor peptide from human His6-tagged RORgammat LBD (264 to 518 residues) by TR-FRET assay | J Med Chem 62: 10816-10832 (2019) Article DOI: 10.1021/acs.jmedchem.9b01291 BindingDB Entry DOI: 10.7270/Q2CF9TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM116236 (US10035794, Example 11 | US10683287, Example 11 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type APP751 (unknown origin) expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM134401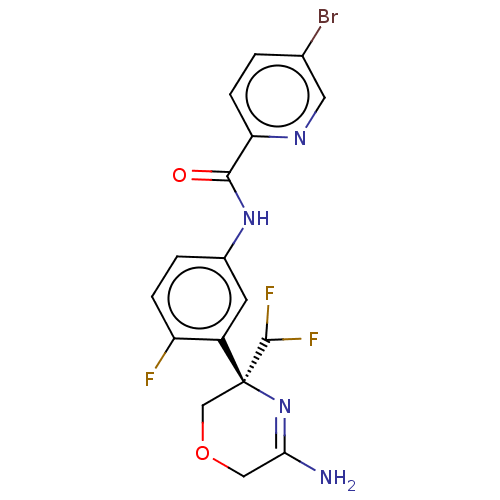 (US8846658, 118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... | Bioorg Med Chem Lett 28: 2195-2200 (2018) Article DOI: 10.1016/j.bmcl.2018.05.003 BindingDB Entry DOI: 10.7270/Q26T0Q5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM116257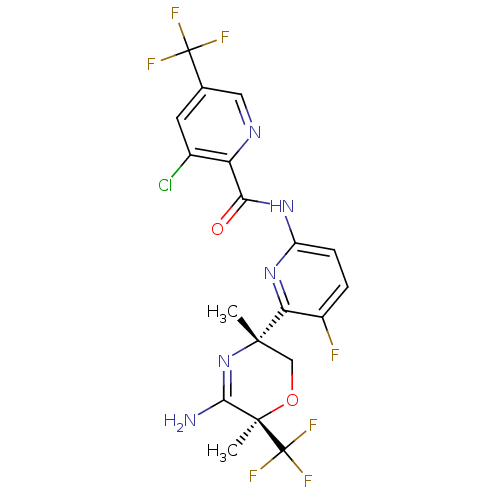 (US10035794, Example 34 | US10683287, Example 34 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type APP751 (unknown origin) expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM116257 (US10035794, Example 34 | US10683287, Example 34 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells assessed as reduction in amyloid beta42 level | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM116257 (US10035794, Example 34 | US10683287, Example 34 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells assessed as reduction in amyloid beta(1 to 40 residues) level | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM116257 (US10035794, Example 34 | US10683287, Example 34 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well i... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM134356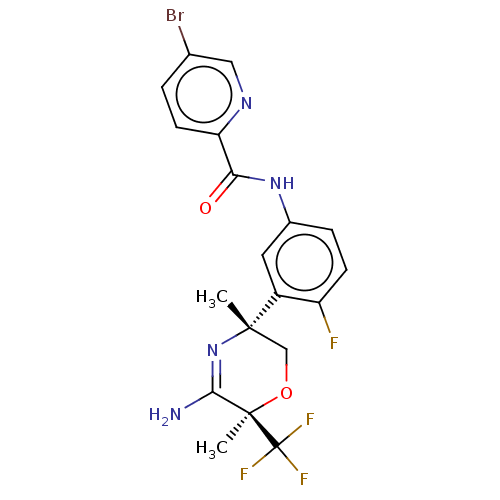 (US8846658, 68) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM134359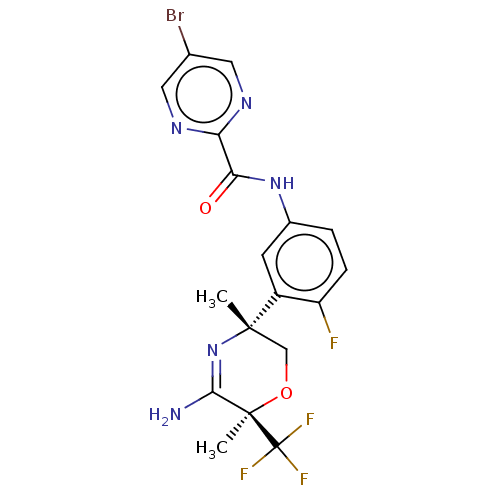 (US8846658, 71) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM134348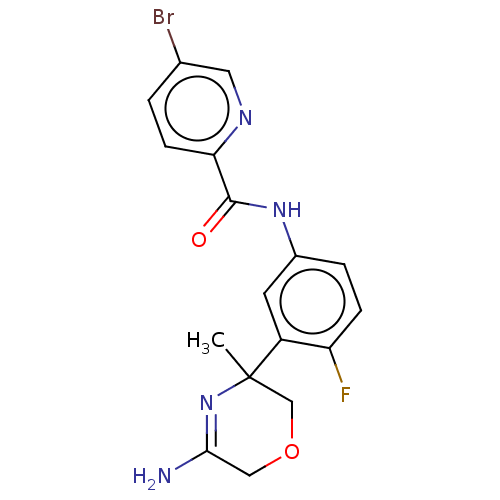 (US8846658, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP expressed in CHO cells assessed as reduction in amyloid beta 40 secretion incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP expressed in CHO cells assessed as reduction in amyloid beta 42 secretion incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50273411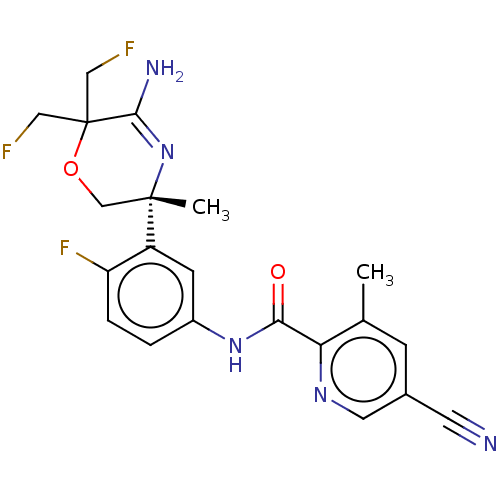 (CHEMBL4127572) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... | Bioorg Med Chem Lett 28: 2195-2200 (2018) Article DOI: 10.1016/j.bmcl.2018.05.003 BindingDB Entry DOI: 10.7270/Q26T0Q5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM116247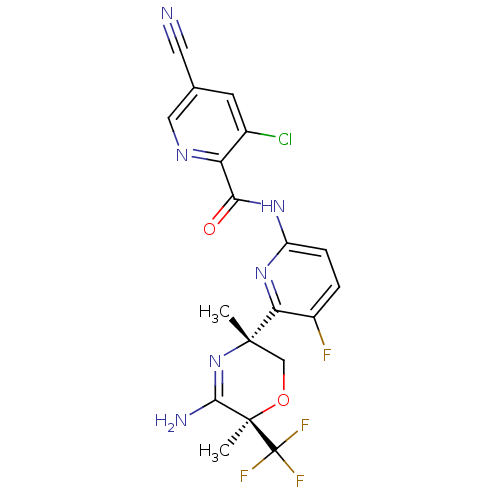 (US10035794, Example 24 | US10683287, Example 35 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well i... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM116247 (US10035794, Example 24 | US10683287, Example 35 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type APP751 (unknown origin) expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM116257 (US10035794, Example 34 | US10683287, Example 34 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells assessed as reduction in amyloid beta40 level | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116236 (US10035794, Example 11 | US10683287, Example 11 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM134358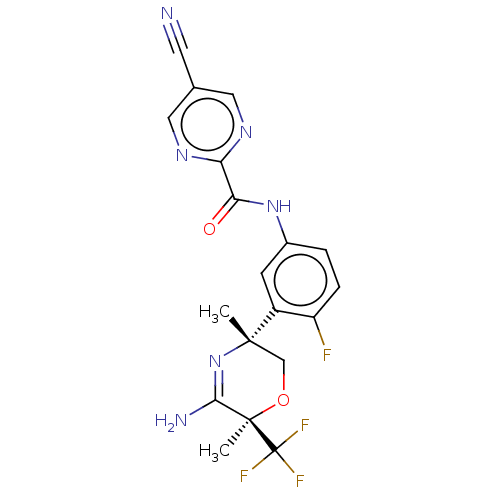 (US8846658, 70) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50587071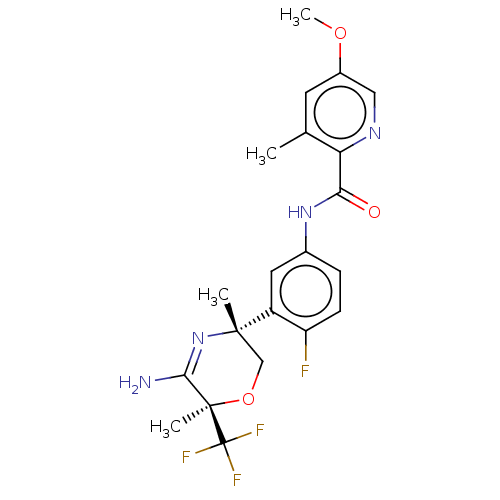 (CHEMBL5075177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP expressed in CHO cells assessed as reduction in soluble APP beta level incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50587069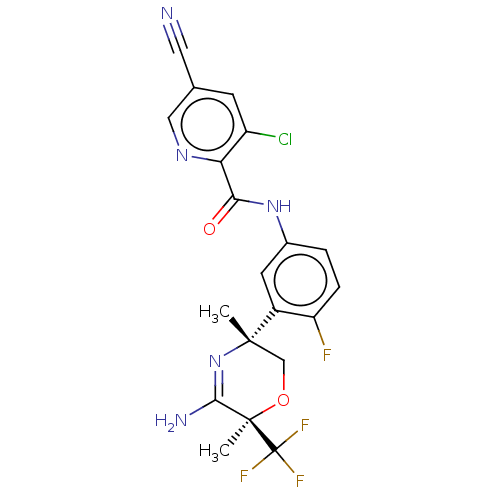 (CHEMBL5073160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50587070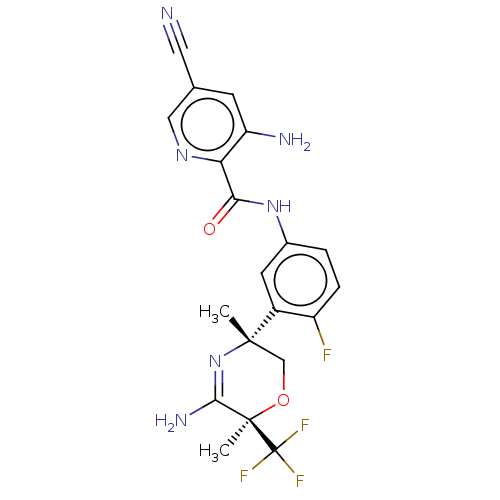 (CHEMBL5090563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50273421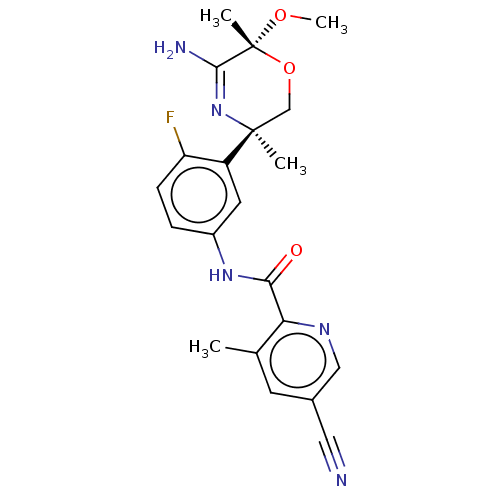 (CHEMBL4126351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... | Bioorg Med Chem Lett 28: 2195-2200 (2018) Article DOI: 10.1016/j.bmcl.2018.05.003 BindingDB Entry DOI: 10.7270/Q26T0Q5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116236 (US10035794, Example 11 | US10683287, Example 11 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10683287 (2020) BindingDB Entry DOI: 10.7270/Q2DZ0CCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50506053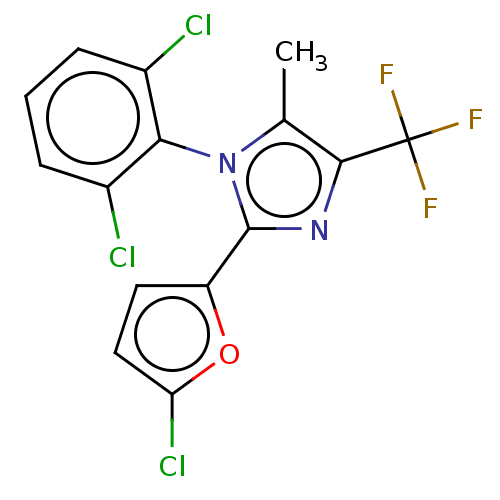 (CHEMBL4562740) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Discovery Chemistry Curated by ChEMBL | Assay Description Displacement of RIP140 cofactor peptide from human His6-tagged RORgammat LBD (264 to 518 residues) by TR-FRET assay | J Med Chem 62: 10816-10832 (2019) Article DOI: 10.1021/acs.jmedchem.9b01291 BindingDB Entry DOI: 10.7270/Q2CF9TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50506052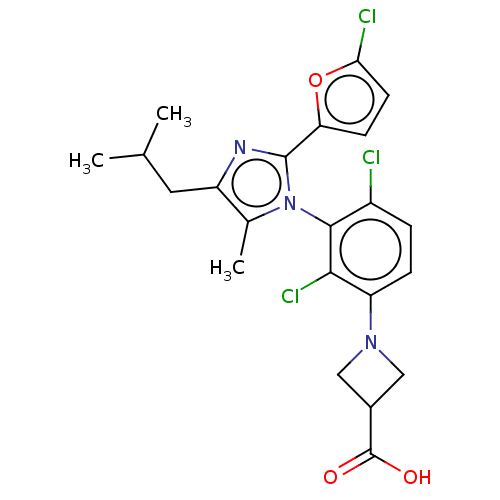 (CHEMBL4446191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Discovery Chemistry Curated by ChEMBL | Assay Description Displacement of RIP140 cofactor peptide from human His6-tagged RORgammat LBD (264 to 518 residues) by TR-FRET assay | J Med Chem 62: 10816-10832 (2019) Article DOI: 10.1021/acs.jmedchem.9b01291 BindingDB Entry DOI: 10.7270/Q2CF9TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50506050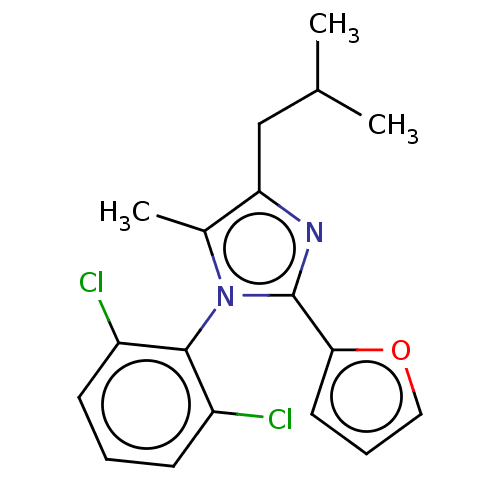 (CHEMBL4580481) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Discovery Chemistry Curated by ChEMBL | Assay Description Displacement of RIP140 cofactor peptide from human His6-tagged RORgammat LBD (264 to 518 residues) by TR-FRET assay | J Med Chem 62: 10816-10832 (2019) Article DOI: 10.1021/acs.jmedchem.9b01291 BindingDB Entry DOI: 10.7270/Q2CF9TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50273406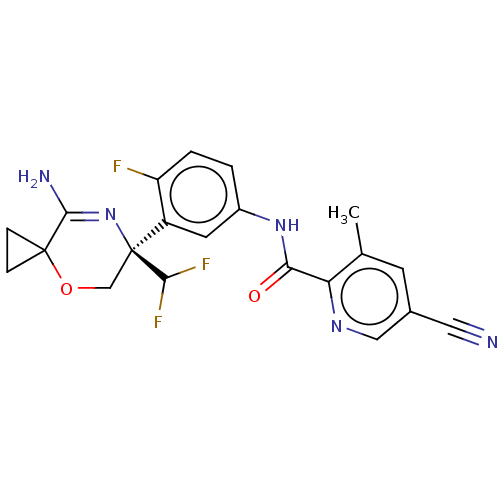 (CHEMBL4128406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... | Bioorg Med Chem Lett 28: 2195-2200 (2018) Article DOI: 10.1016/j.bmcl.2018.05.003 BindingDB Entry DOI: 10.7270/Q26T0Q5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM134322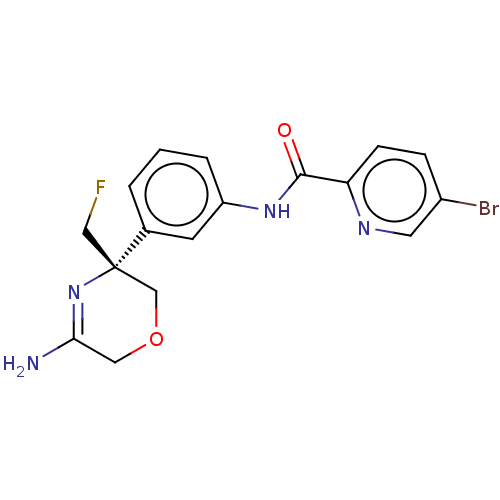 (US8846658, 33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in CHO cells co-expressing human APP751 assessed as decrease in amyloid beta 40 levels after 24 hrs by... | Bioorg Med Chem Lett 28: 2195-2200 (2018) Article DOI: 10.1016/j.bmcl.2018.05.003 BindingDB Entry DOI: 10.7270/Q26T0Q5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116236 (US10035794, Example 11 | US10683287, Example 11 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US8637508 (2014) BindingDB Entry DOI: 10.7270/Q2JD4VFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50587067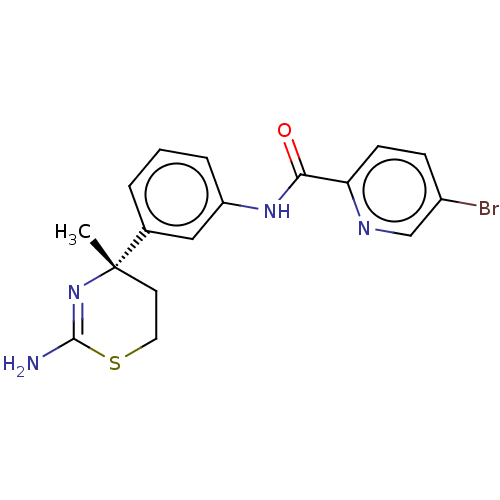 (CHEMBL5093426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of APP751 (unknown origin) expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate with BACE-cleavable sequence | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116233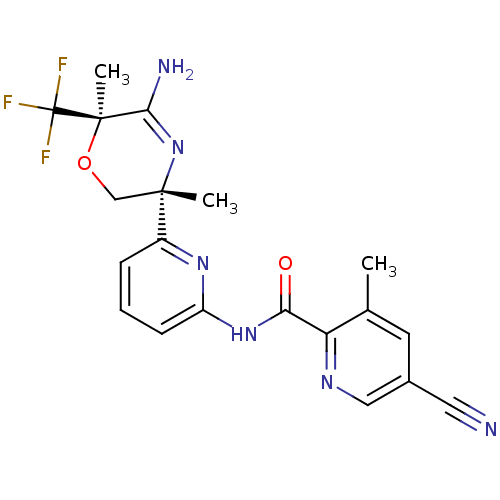 (US10035794, Example 8 | US10683287, Example 8 | US...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM116244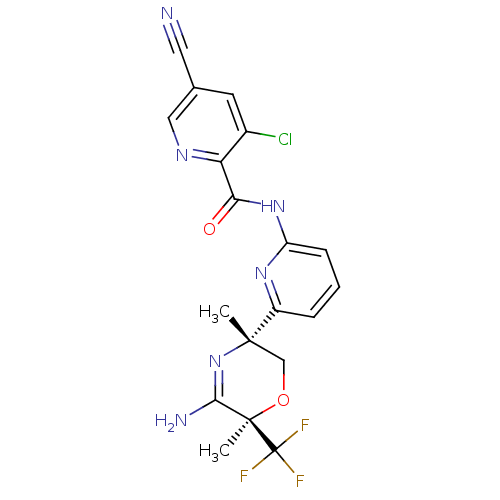 (US10035794, Example 20 | US10683287, Example 20 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well i... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE1 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50587070 (CHEMBL5090563) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE1 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50587069 (CHEMBL5073160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50587070 (CHEMBL5090563) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM134359 (US8846658, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type human APP751 expressed in CHO cells incubated for 24 hrs by immunoassay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE1 catalytic domain using FRET substrate with BACE-cleavable sequence | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116236 (US10035794, Example 11 | US10683287, Example 11 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE1 catalytic domain using FRET substrate with BACE-cleavable sequence | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM116233 (US10035794, Example 8 | US10683287, Example 8 | US...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Recombinant BACE-1 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10683287 (2020) BindingDB Entry DOI: 10.7270/Q2DZ0CCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 651 total ) | Next | Last >> |