 Found 11663 hits with Last Name = 'walls' and Initial = 's'
Found 11663 hits with Last Name = 'walls' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364234
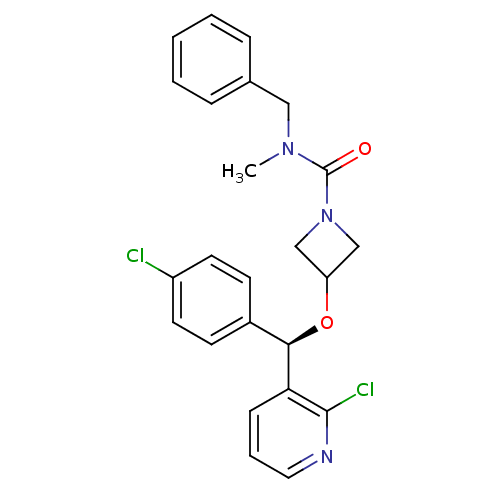
(CHEMBL1952279)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50296485
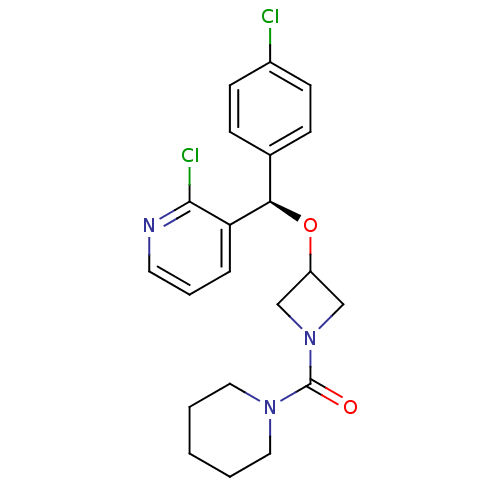
((R)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364235
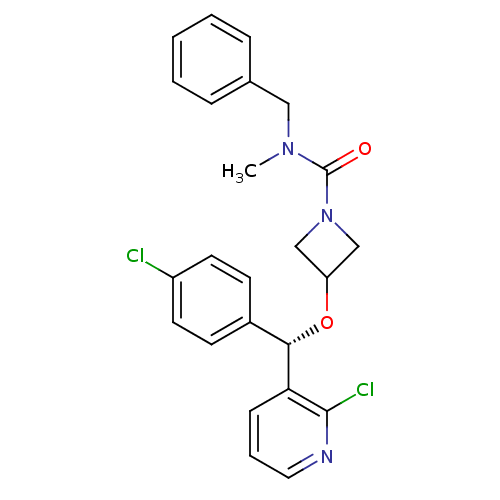
(CHEMBL1952280)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364234

(CHEMBL1952279)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50296484
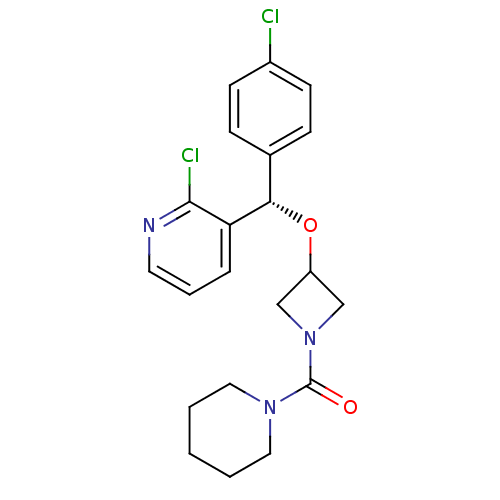
((S)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 19: 4241-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.097
BindingDB Entry DOI: 10.7270/Q28S4PZC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364235

(CHEMBL1952280)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50296484

((S)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 19: 4241-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.097
BindingDB Entry DOI: 10.7270/Q28S4PZC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50296485

((R)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 19: 4241-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.097
BindingDB Entry DOI: 10.7270/Q28S4PZC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50296485

((R)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [G810S]
(Homo sapiens (Human)) | BDBM576988
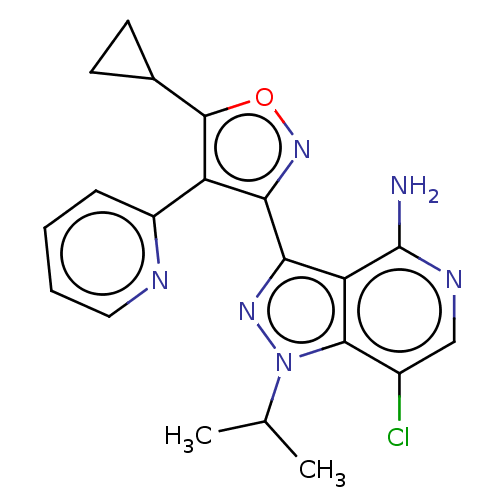
(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577053

(US11472802, Example 58)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)nccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [G810R]
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577053

(US11472802, Example 58)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)nccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577063
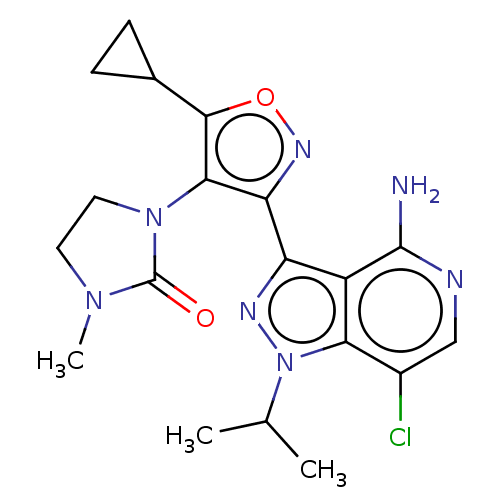
(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCN(C)C2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577046
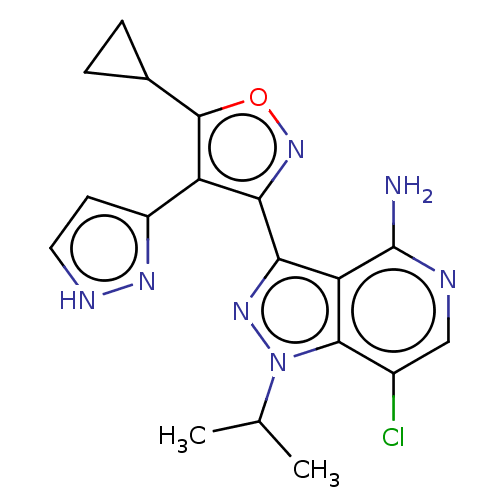
(7-chloro-3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)iso...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577050

(7-chloro-3-(5- cyclopropyl-4-(1H- imidazol-4- yl)i...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577049

(US11472802, Example 55)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577049

(US11472802, Example 55)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577046

(7-chloro-3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)iso...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM577049

(US11472802, Example 55)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577063

(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCN(C)C2=O)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577061

(US11472802, Example 65)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCC2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577061

(US11472802, Example 65)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCC2=O)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM576996

(US11472802, Example 11)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577026

(7-chloro-3-(5-cyclopropyl-4-(4- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)ccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577025
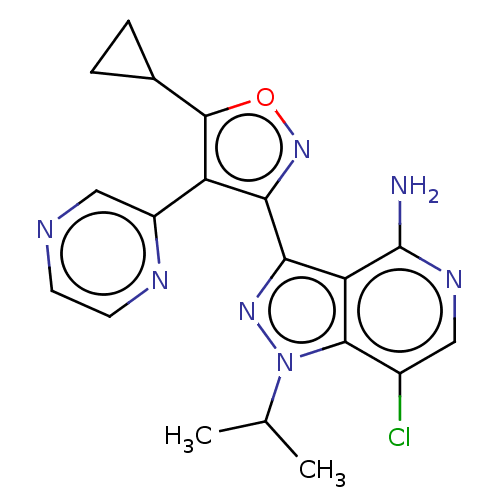
(7-chloro-3-(5-cyclopropyl-4- (pyrazin-2-yl)isoxazo...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cnccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577045

(US11472802, Example 51)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577066
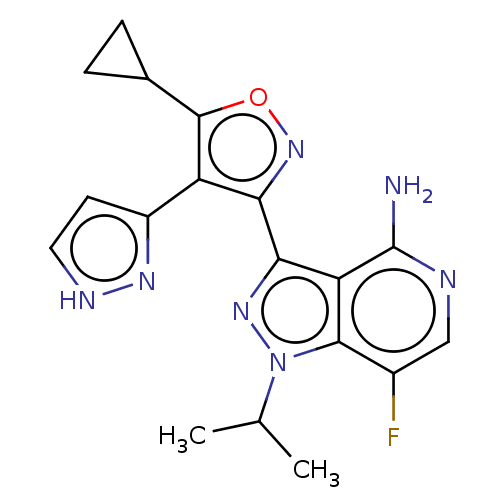
(3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)isoxazol-3- ...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577064
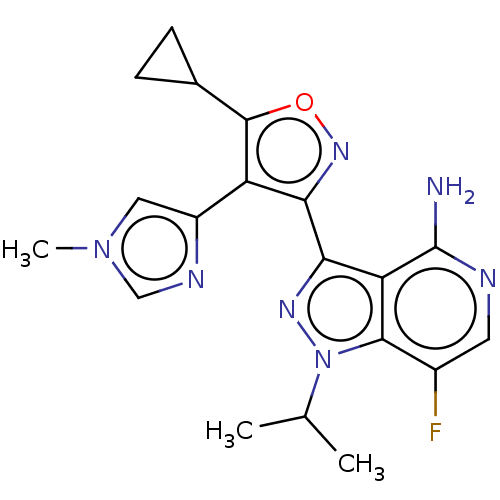
(US11472802, Example 68)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577064

(US11472802, Example 68)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577050

(7-chloro-3-(5- cyclopropyl-4-(1H- imidazol-4- yl)i...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM576996

(US11472802, Example 11)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577067
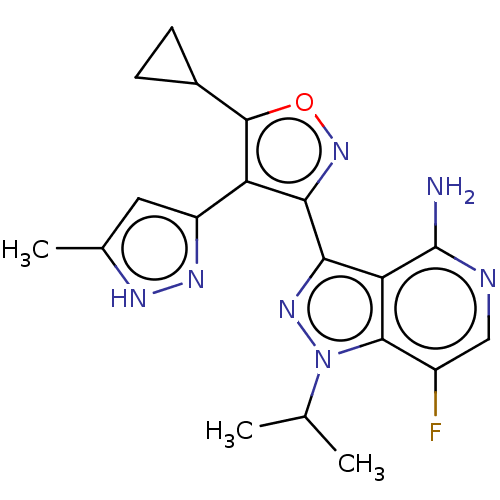
(3-(5-cyclopropyl- 4-(5-methyl-1H- pyrazol-3-yl) is...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577066

(3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)isoxazol-3- ...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM576996

(US11472802, Example 11)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM577063

(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCN(C)C2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577062
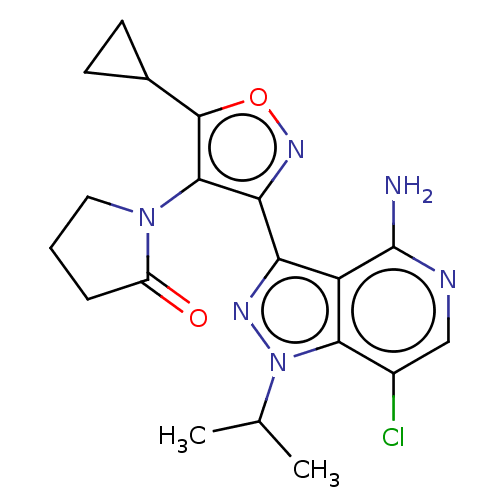
(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCCC2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577045

(US11472802, Example 51)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577029

(7-chloro-3-(5-cyclopropyl-4-(5- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccc(C)cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577025

(7-chloro-3-(5-cyclopropyl-4- (pyrazin-2-yl)isoxazo...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cnccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577026

(7-chloro-3-(5-cyclopropyl-4-(4- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)ccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577048

(7-chloro-3-(5-cyclopropyl- 4-(1-ethyl-1H-pyrazol-3...)Show SMILES CCn1ccc(n1)-c1c(noc1C1CC1)-c1nn(C(C)C)c2c(Cl)cnc(N)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577047

(7-chloro-3-(5-cyclopropyl- 4-(1-methyl-1H-pyrazol-...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccn(C)n2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM577064

(US11472802, Example 68)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577067

(3-(5-cyclopropyl- 4-(5-methyl-1H- pyrazol-3-yl) is...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577029

(7-chloro-3-(5-cyclopropyl-4-(5- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccc(C)cn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577062

(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCCC2=O)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data