

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021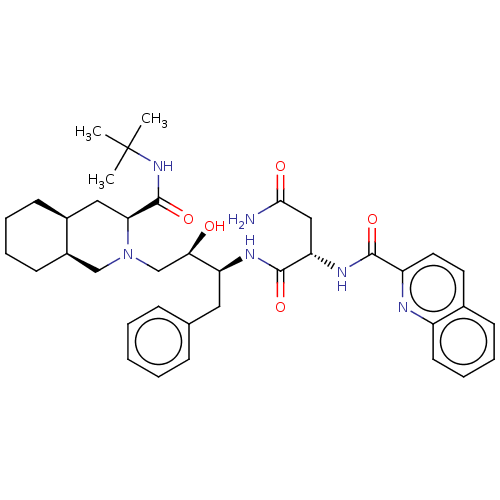 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593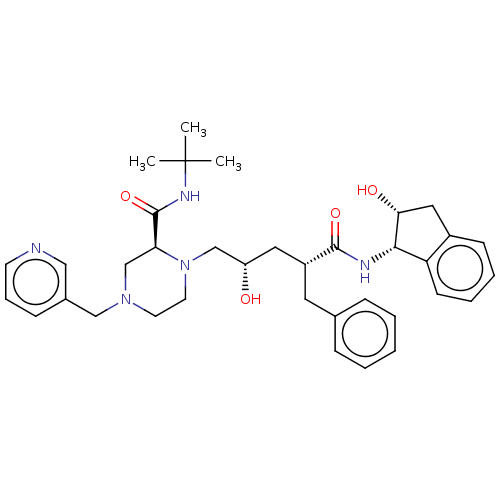 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50088504 (A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480942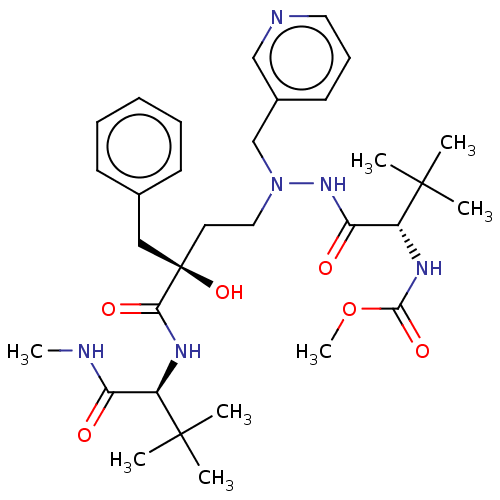 (CHEMBL583069) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480946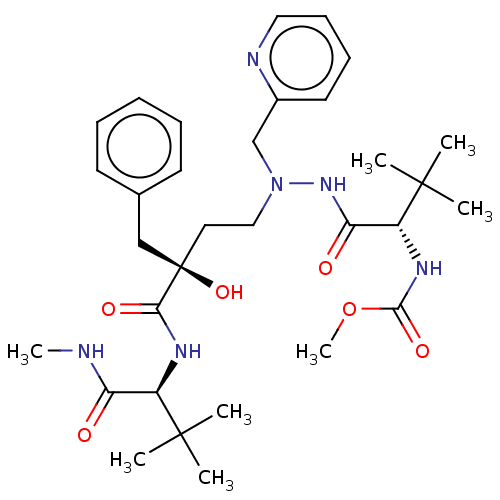 (CHEMBL583568) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480943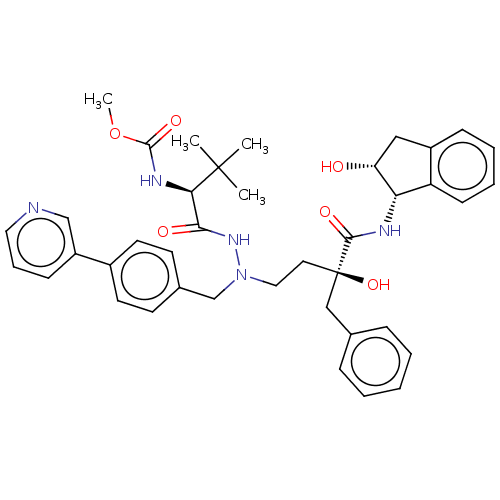 (CHEMBL583371) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480938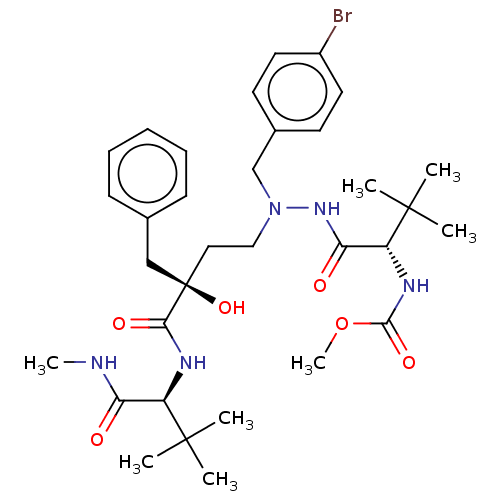 (CHEMBL574797) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480933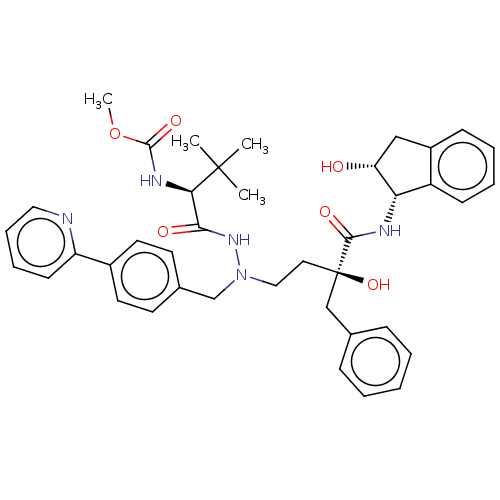 (CHEMBL583370) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480949 (CHEMBL574755) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480936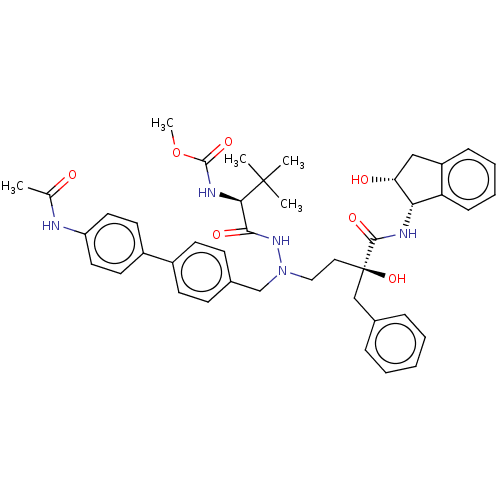 (CHEMBL574733) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93250 (HIV-1 Protease Inhibitor, 7g) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480950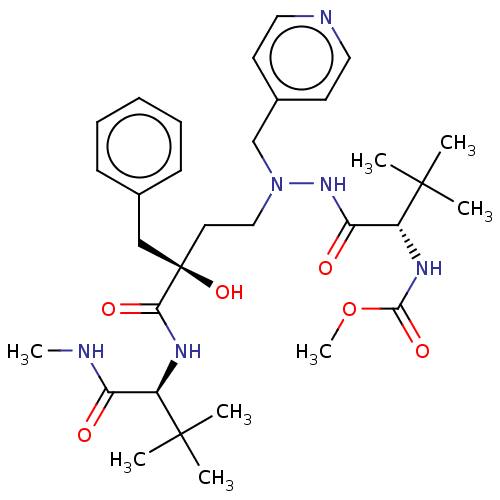 (CHEMBL583070) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480953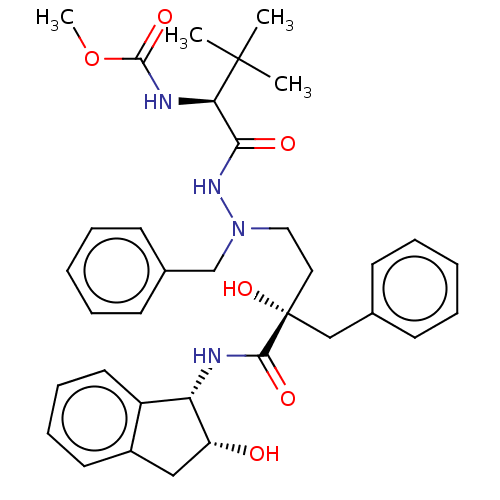 (CHEMBL583565) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480934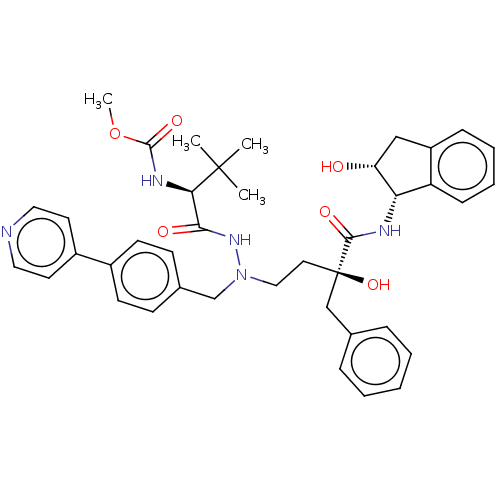 (CHEMBL583372) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480937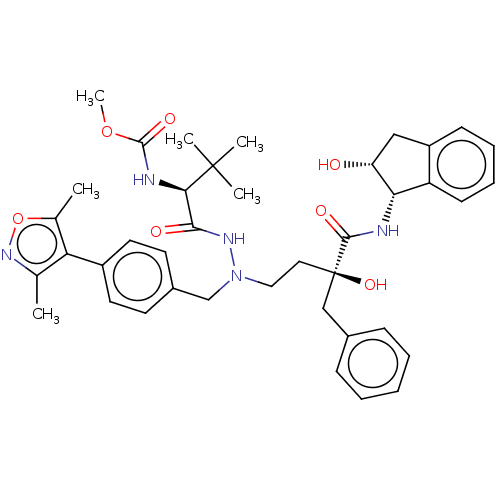 (CHEMBL583564) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478118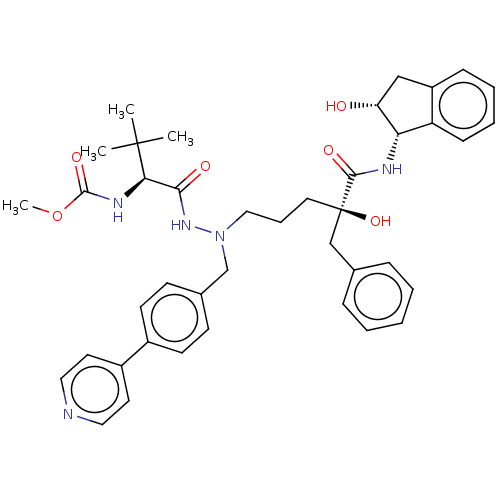 (CHEMBL270135) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480944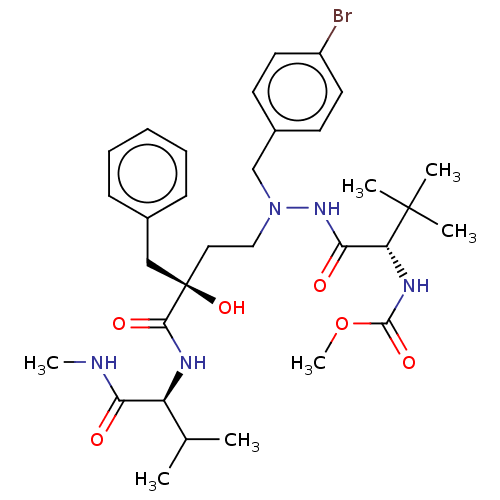 (CHEMBL574743) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480932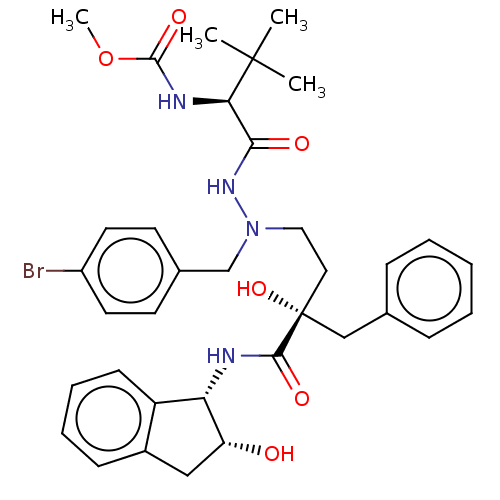 (CHEMBL574795) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93250 (HIV-1 Protease Inhibitor, 7g) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93245 (HIV-1 Protease Inhibitor, 7b) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480940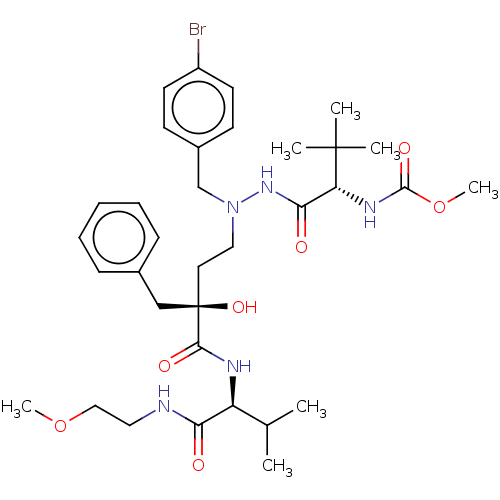 (CHEMBL574751) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50183627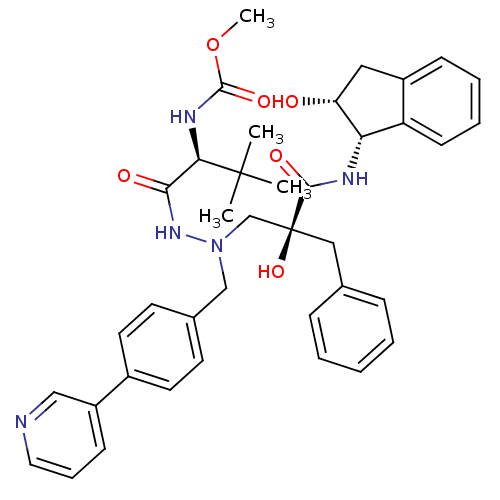 (CHEMBL206467 | methyl(S)-1-(2-((S)-2-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93241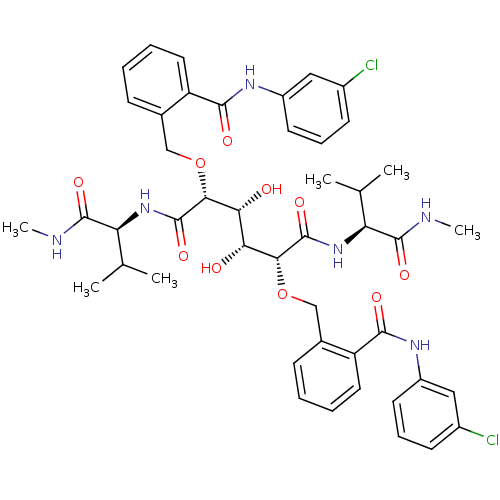 (HIV-1 Protease Inhibitor, 6l) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93245 (HIV-1 Protease Inhibitor, 7b) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549529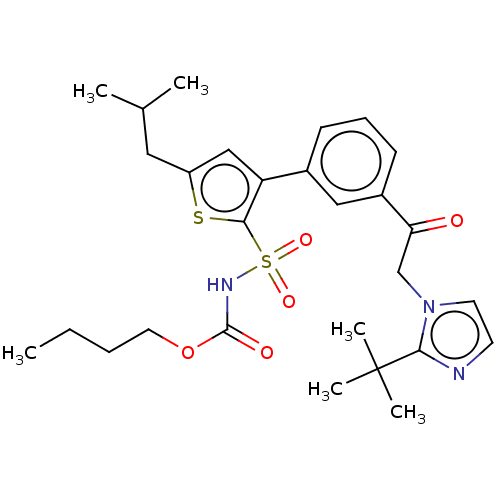 (CHEMBL4779819) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93237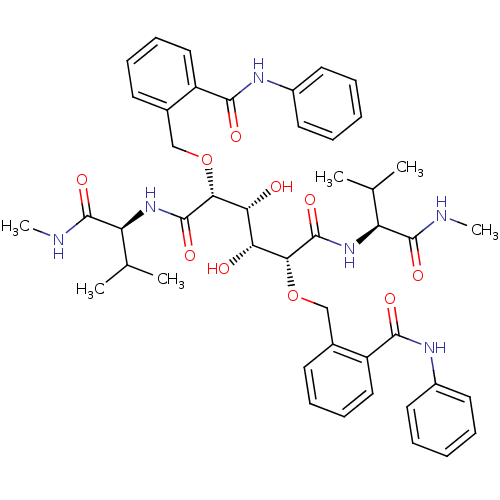 (HIV-1 Protease Inhibitor, 6h) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93249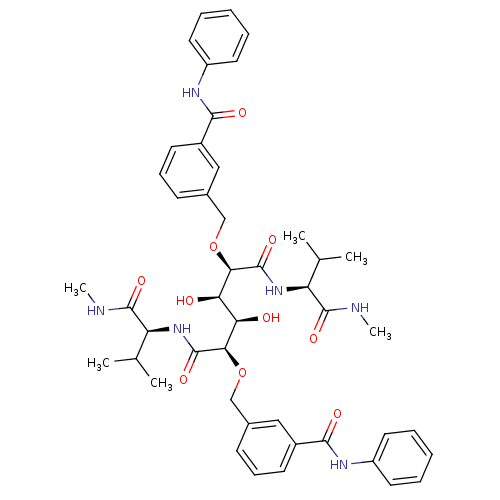 (HIV-1 Protease Inhibitor, 7f) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549533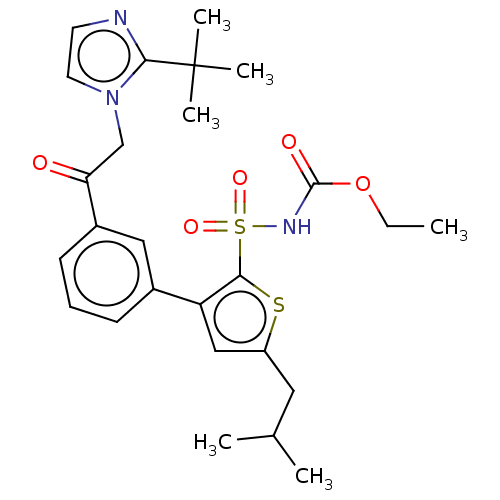 (CHEMBL4779945) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480935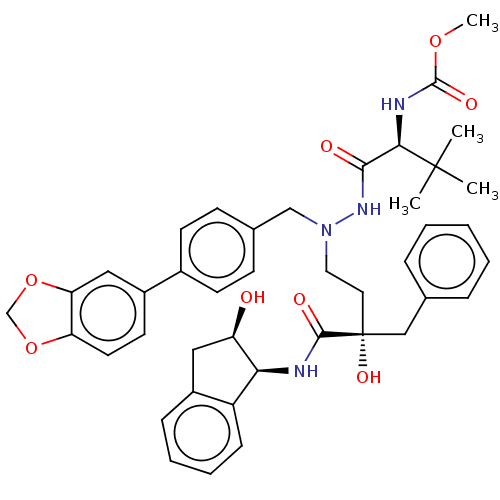 (CHEMBL574796) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480945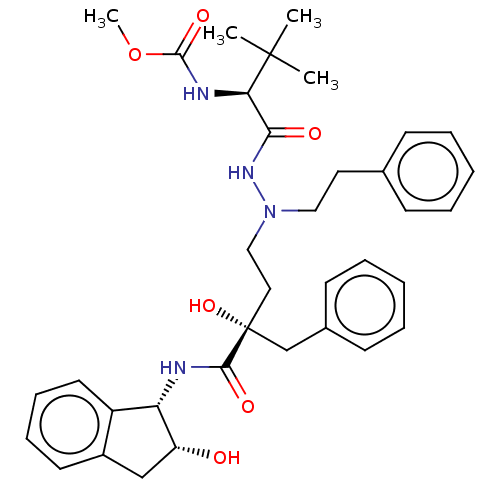 (CHEMBL583566) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93241 (HIV-1 Protease Inhibitor, 6l) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549530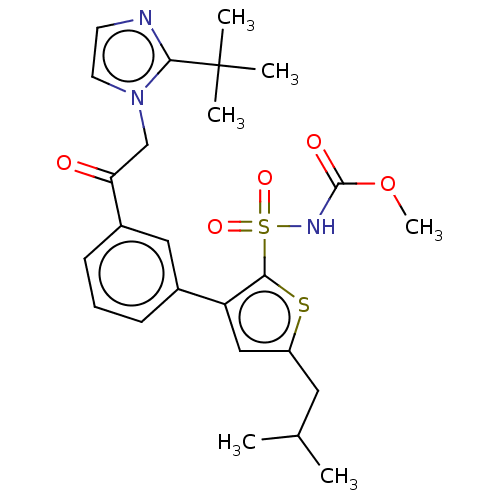 (CHEMBL4788892) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480948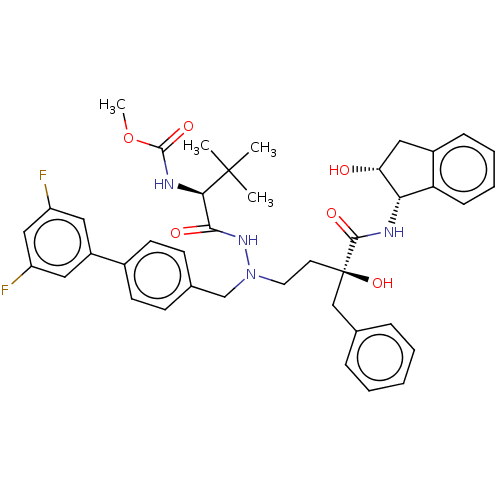 (CHEMBL583563) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480952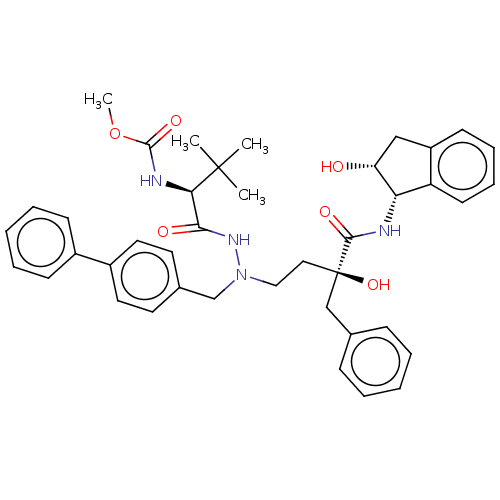 (CHEMBL583369) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50480933 (CHEMBL583370) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease L63P, V82T, I84V mutant | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50480943 (CHEMBL583371) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease L63P, V82T, I84V mutant | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549528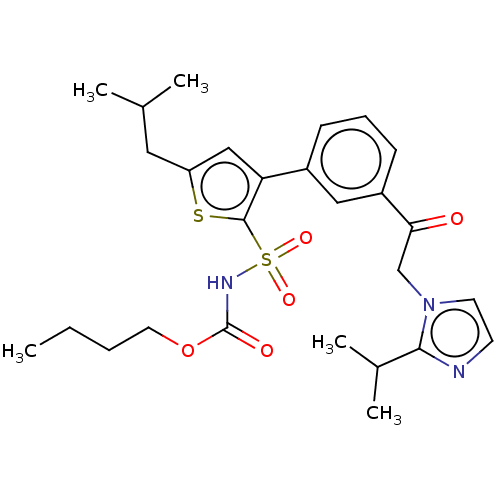 (CHEMBL4759641) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93244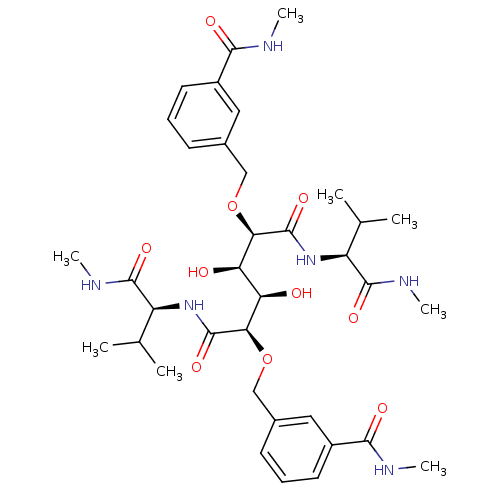 (HIV-1 Protease Inhibitor, 7a) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93237 (HIV-1 Protease Inhibitor, 6h) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93248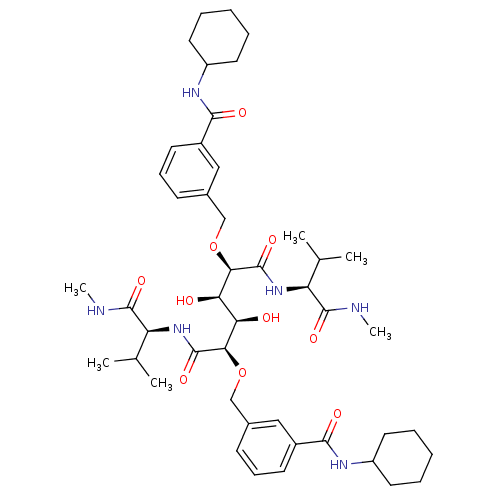 (HIV-1 Protease Inhibitor, 7e) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549526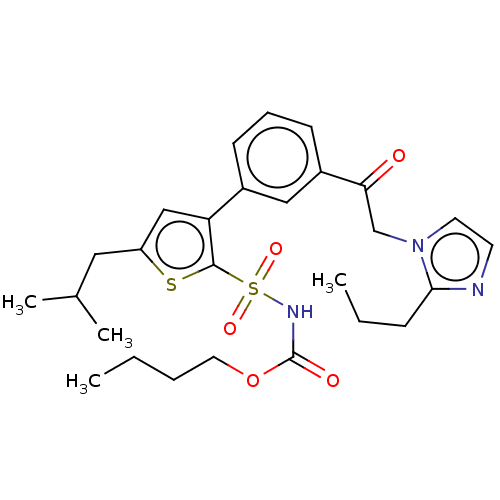 (CHEMBL4751226) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50480932 (CHEMBL574795) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease L63P, V82T, I84V mutant | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549527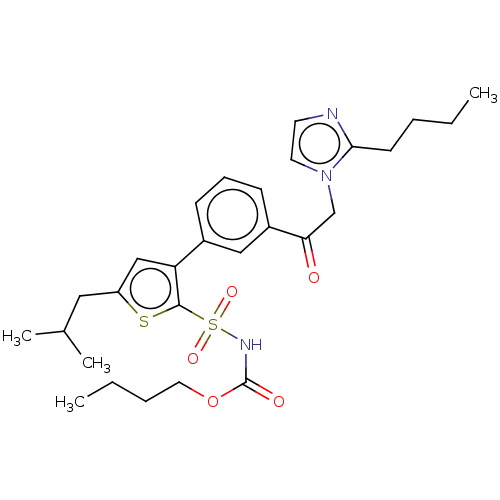 (CHEMBL4784233) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549525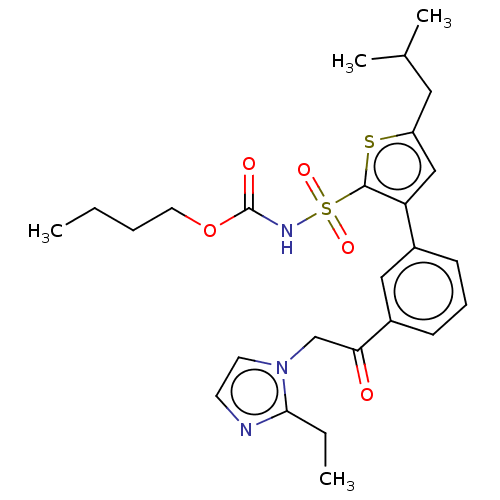 (CHEMBL2086911) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549532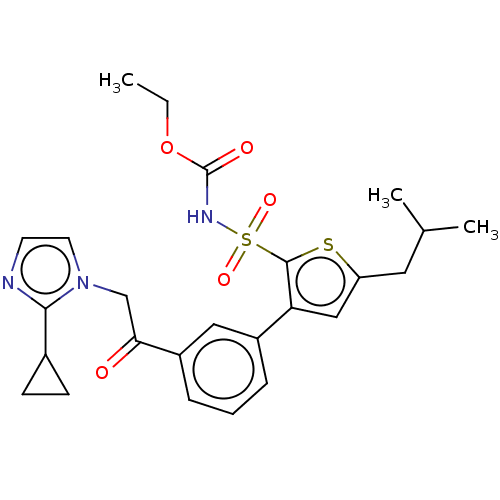 (CHEMBL4747530) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM93238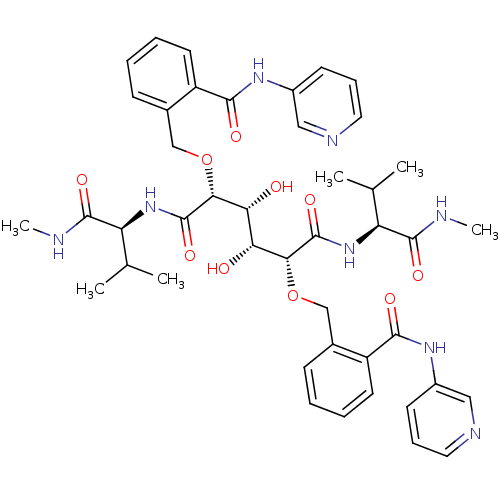 (HIV-1 Protease Inhibitor, 6i) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Fluorometric assay using HIV-1 protease. | J Comb Chem 7: 611-7 (2005) Article DOI: 10.1021/cc050016r BindingDB Entry DOI: 10.7270/Q2GQ6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549531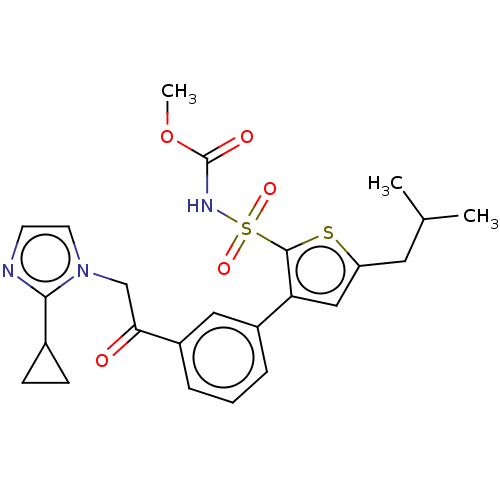 (CHEMBL4762752) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |