 Found 2251 hits with Last Name = 'waterson' and Initial = 'ag'
Found 2251 hits with Last Name = 'waterson' and Initial = 'ag' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
GTPase KRas
(Homo sapiens (Human)) | BDBM50607576
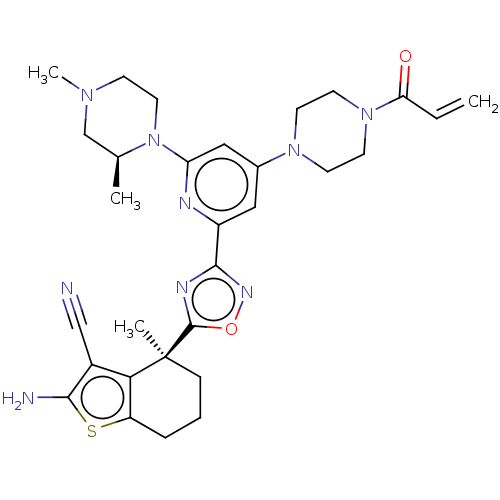
(CHEMBL5218642)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(n1)-c1noc(n1)[C@@]1(C)CCCc2sc(N)c(C#N)c12)N1CCN(CC1)C(=O)C=C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01120
BindingDB Entry DOI: 10.7270/Q2CC14S5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50607575

(CHEMBL5221087)Show SMILES C[C@@]1(CCCc2sc(N)c(C#N)c12)c1nc(no1)-c1cccc(c1)N1CCN(CC1)C(=O)C=C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01120
BindingDB Entry DOI: 10.7270/Q2CC14S5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50607574
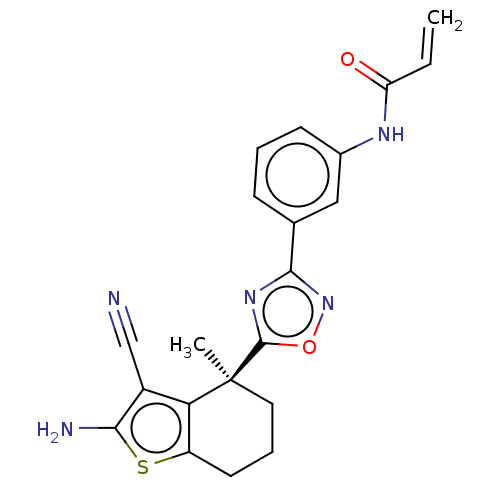
(CHEMBL5218841)Show SMILES C[C@@]1(CCCc2sc(N)c(C#N)c12)c1nc(no1)-c1cccc(NC(=O)C=C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01120
BindingDB Entry DOI: 10.7270/Q2CC14S5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50514402
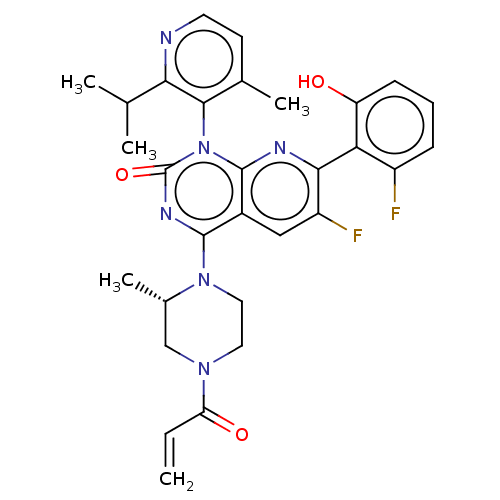
(CHEMBL4535757 | US11345701, Compound Amg-510)Show SMILES CC(C)c1nccc(C)c1-n1c2nc(c(F)cc2c(nc1=O)N1CCN(C[C@@H]1C)C(=O)C=C)-c1c(O)cccc1F |r,wU:27.31,(31.19,-31.19,;32.52,-31.96,;32.53,-33.49,;33.85,-31.18,;35.18,-31.95,;36.51,-31.17,;36.5,-29.62,;35.16,-28.87,;35.15,-27.33,;33.84,-29.64,;32.51,-28.88,;32.5,-27.35,;33.85,-26.57,;33.84,-25.02,;32.5,-24.25,;32.5,-22.71,;31.17,-25.02,;31.17,-26.57,;29.84,-27.34,;29.84,-28.87,;31.17,-29.64,;29.83,-30.4,;28.51,-26.56,;28.52,-25.02,;27.2,-24.24,;25.86,-25,;25.85,-26.54,;27.19,-27.33,;27.18,-28.87,;24.53,-24.22,;24.54,-22.68,;23.19,-24.98,;21.85,-24.2,;35.17,-24.24,;36.51,-25.01,;36.51,-26.55,;37.84,-24.24,;37.84,-22.69,;36.49,-21.92,;35.16,-22.71,;33.82,-21.94,)| Show InChI InChI=1S/C30H30F2N6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-21(32)26(24-20(31)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01120
BindingDB Entry DOI: 10.7270/Q2CC14S5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50607573
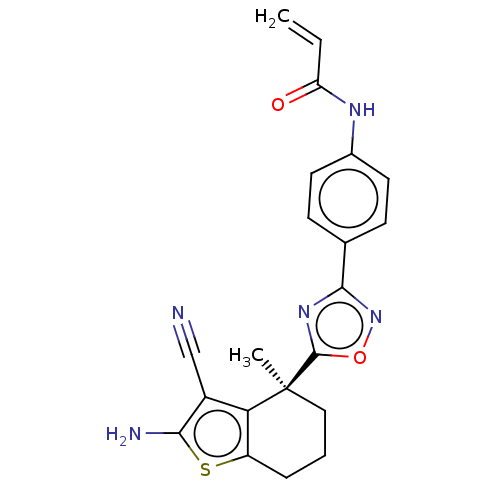
(CHEMBL5219918)Show SMILES C[C@@]1(CCCc2sc(N)c(C#N)c12)c1nc(no1)-c1ccc(NC(=O)C=C)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01120
BindingDB Entry DOI: 10.7270/Q2CC14S5 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546980

(CHEMBL4792513)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(F)C2CCOC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50507325

(CHEMBL4453586)Show SMILES C[C@@H](CN1CCC2(CC1)[C@@H](CNC2=O)c1ccc(F)cc1)NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H29FN4O2/c1-17(29-24(32)23-14-19-4-2-3-5-22(19)30-23)16-31-12-10-26(11-13-31)21(15-28-25(26)33)18-6-8-20(27)9-7-18/h2-9,14,17,21,30H,10-13,15-16H2,1H3,(H,28,33)(H,29,32)/t17-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu1 cells |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM50507332

(CHEMBL4475943)Show SMILES Fc1ccc(cc1)[C@@H]1CNC(=O)C11CCN(CCNC(=O)c2cc3ccccc3[nH]2)CC1 |r| Show InChI InChI=1S/C25H27FN4O2/c26-19-7-5-17(6-8-19)20-16-28-24(32)25(20)9-12-30(13-10-25)14-11-27-23(31)22-15-18-3-1-2-4-21(18)29-22/h1-8,15,20,29H,9-14,16H2,(H,27,31)(H,28,32)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of GFP-fused PLD2 (unknown origin) expressed in HEK293 cells by cellular assay |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546969
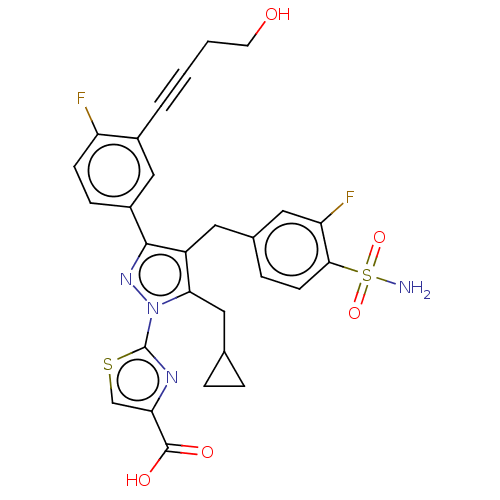
(CHEMBL4786682 | US11247971, Cmpd ID 409)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CCCO)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50257541
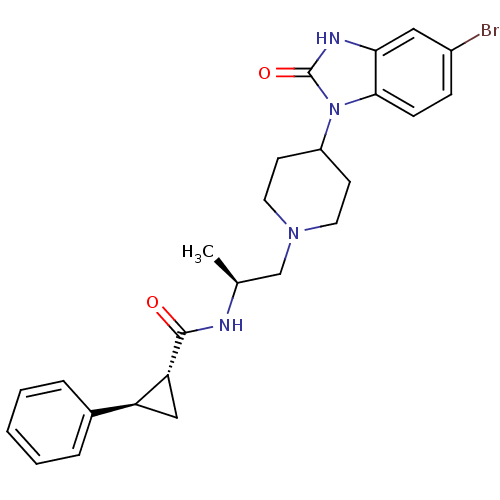
((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...)Show SMILES C[C@@H](CN1CCC(CC1)n1c2ccc(Br)cc2[nH]c1=O)NC(=O)[C@@H]1C[C@H]1c1ccccc1 |r| Show InChI InChI=1S/C25H29BrN4O2/c1-16(27-24(31)21-14-20(21)17-5-3-2-4-6-17)15-29-11-9-19(10-12-29)30-23-8-7-18(26)13-22(23)28-25(30)32/h2-8,13,16,19-21H,9-12,14-15H2,1H3,(H,27,31)(H,28,32)/t16-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM537077

(US11247971, Cmpd ID 400)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CC3(F)F)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546981

(CHEMBL4797357)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(O)C2CCOC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489090

(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CCCO3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C29H28F2N4O6S2/c30-21-7-6-18(13-25(21)41-14-19-2-1-9-40-19)27-20(10-17-5-8-26(22(31)11-17)43(32,38)39)24(12-16-3-4-16)35(34-27)29-33-23(15-42-29)28(36)37/h5-8,11,13,15-16,19H,1-4,9-10,12,14H2,(H,36,37)(H2,32,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489091

(2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OCC3CCOC3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C29H28F2N4O6S2/c30-21-5-4-19(12-25(21)41-14-18-7-8-40-13-18)27-20(9-17-3-6-26(22(31)10-17)43(32,38)39)24(11-16-1-2-16)35(34-27)29-33-23(15-42-29)28(36)37/h3-6,10,12,15-16,18H,1-2,7-9,11,13-14H2,(H,36,37)(H2,32,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM154517

(ML299 (5))Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(F)c1)NC(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C23H26BrFN4O2/c1-16(27-21(30)17-5-7-18(24)8-6-17)14-28-11-9-23(10-12-28)22(31)26-15-29(23)20-4-2-3-19(25)13-20/h2-8,13,16H,9-12,14-15H2,1H3,(H,26,31)(H,27,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 (unknown origin) |
Bioorg Med Chem Lett 28: 3670-3673 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.033
BindingDB Entry DOI: 10.7270/Q2HQ436Z |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546978
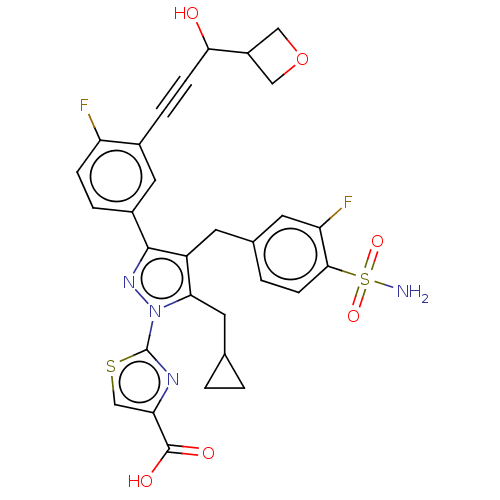
(CHEMBL4752940)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(O)C2COC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489160

(2-(3-(3-(tert- butylcarbamoyl)-4- fluorophenyl)-5-...)Show SMILES CC(C)(C)NC(=O)c1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O Show InChI InChI=1S/C29H29F2N5O5S2/c1-29(2,3)34-26(37)18-13-17(7-8-20(18)30)25-19(10-16-6-9-24(21(31)11-16)43(32,40)41)23(12-15-4-5-15)36(35-25)28-33-22(14-42-28)27(38)39/h6-9,11,13-15H,4-5,10,12H2,1-3H3,(H,34,37)(H,38,39)(H2,32,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM27973
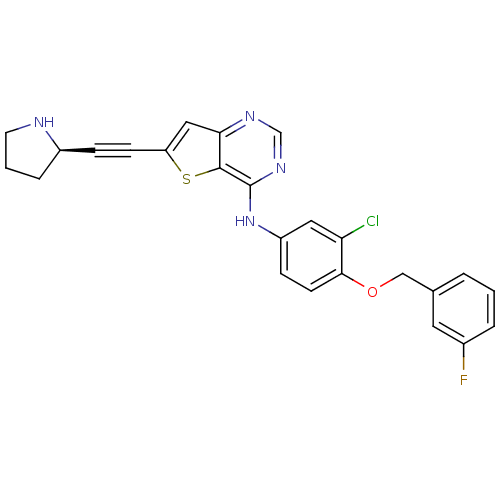
(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM27973

(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546979
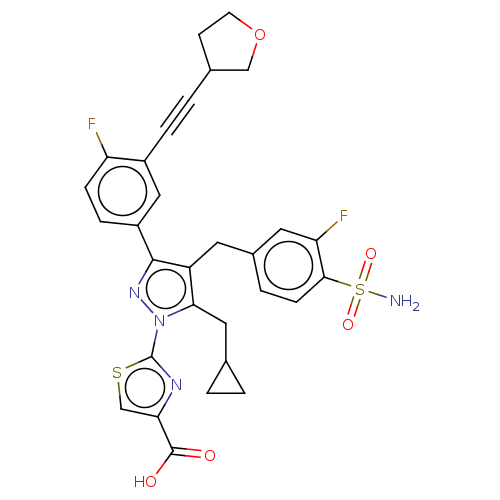
(CHEMBL4747300 | US11247971, Cmpd ID 423)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC2CCOC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546975
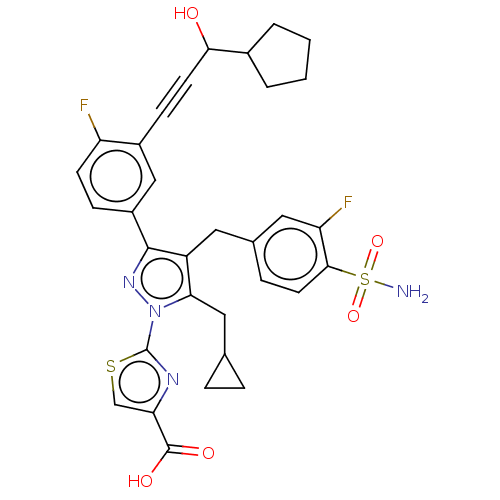
(CHEMBL4749903)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(O)C2CCCC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546977
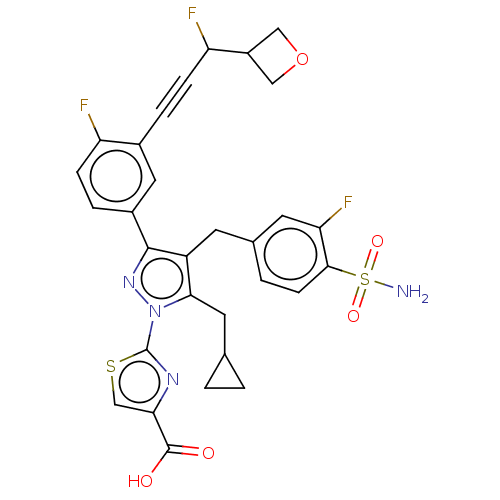
(CHEMBL4759378)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(F)C2COC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50569440

(CHEMBL4877988 | US11752138, Compound 152)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(NC(=O)c3ccccc3)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50250655
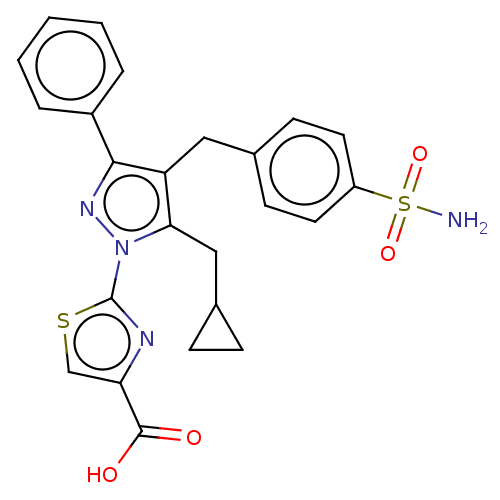
(CHEMBL4059985 | US10961200, Compound 189 | US11247...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C24H22N4O4S2/c25-34(31,32)18-10-8-15(9-11-18)12-19-21(13-16-6-7-16)28(24-26-20(14-33-24)23(29)30)27-22(19)17-4-2-1-3-5-17/h1-5,8-11,14,16H,6-7,12-13H2,(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes LDHB using sodium pyruvate as substrate after 5 mins in presence of NAPDH by diaphorase/resazurin based fluorescence... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM536955

(US11247971, Cmpd ID 278)Show SMILES COc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(cc1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489092

(2-(3-(3- cydopropoxy-4- fluorophenyl)-5- (cyclopro...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(OC3CC3)c2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C27H24F2N4O5S2/c28-19-7-4-16(12-23(19)38-17-5-6-17)25-18(9-15-3-8-24(20(29)10-15)40(30,36)37)22(11-14-1-2-14)33(32-25)27-31-21(13-39-27)26(34)35/h3-4,7-8,10,12-14,17H,1-2,5-6,9,11H2,(H,34,35)(H2,30,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546998

(CHEMBL4790159 | US11247971, Cmpd ID 405)Show SMILES Cn1cncc1C#Cc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546970
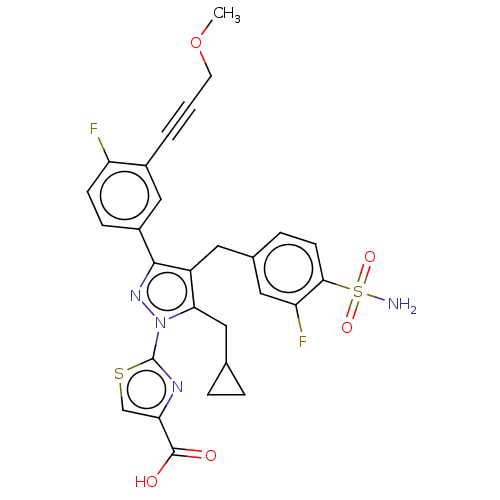
(CHEMBL4783945 | US11247971, Cmpd ID 404)Show SMILES COCC#Cc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546989

(CHEMBL4759499 | US11247971, Cmpd ID 417)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CCN2CCCC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM489161

(2-(3-(3- (benzylcarbamoyl)-4- fluorophenyl)-5- (cy...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C(=O)NCc2ccccc2)-c2nc(cs2)C(O)=O)cc1F Show InChI InChI=1S/C32H27F2N5O5S2/c33-24-10-9-21(15-22(24)30(40)36-16-19-4-2-1-3-5-19)29-23(12-20-8-11-28(25(34)13-20)46(35,43)44)27(14-18-6-7-18)39(38-29)32-37-26(17-45-32)31(41)42/h1-5,8-11,13,15,17-18H,6-7,12,14,16H2,(H,36,40)(H,41,42)(H2,35,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127974
BindingDB Entry DOI: 10.7270/Q24J0JV6 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250655

(CHEMBL4059985 | US10961200, Compound 189 | US11247...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C24H22N4O4S2/c25-34(31,32)18-10-8-15(9-11-18)12-19-21(13-16-6-7-16)28(24-26-20(14-33-24)23(29)30)27-22(19)17-4-2-1-3-5-17/h1-5,8-11,14,16H,6-7,12-13H2,(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50250655

(CHEMBL4059985 | US10961200, Compound 189 | US11247...)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C24H22N4O4S2/c25-34(31,32)18-10-8-15(9-11-18)12-19-21(13-16-6-7-16)28(24-26-20(14-33-24)23(29)30)27-22(19)17-4-2-1-3-5-17/h1-5,8-11,14,16H,6-7,12-13H2,(H,29,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50182908
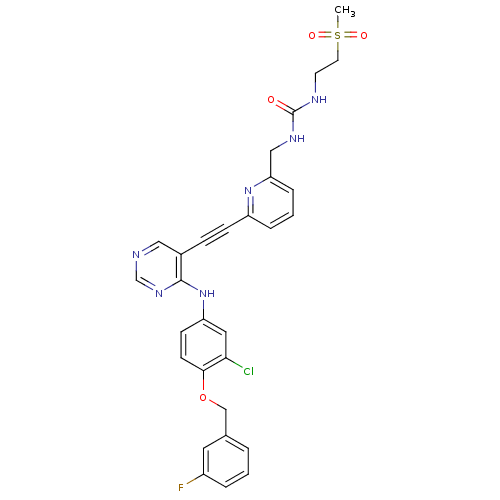
(1-((6-(2-(4-(4-(3-fluorobenzyloxy)-3-chlorophenyla...)Show SMILES CS(=O)(=O)CCNC(=O)NCc1cccc(n1)C#Cc1cncnc1Nc1ccc(OCc2cccc(F)c2)c(Cl)c1 Show InChI InChI=1S/C29H26ClFN6O4S/c1-42(39,40)13-12-33-29(38)34-17-25-7-3-6-23(36-25)9-8-21-16-32-19-35-28(21)37-24-10-11-27(26(30)15-24)41-18-20-4-2-5-22(31)14-20/h2-7,10-11,14-16,19H,12-13,17-18H2,1H3,(H,32,35,37)(H2,33,34,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 2419-22 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.111
BindingDB Entry DOI: 10.7270/Q2JW8DH8 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50547000

(CHEMBL4783252 | US11247971, Cmpd ID 270)Show SMILES CC#Cc1cccc(c1)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546963

(CHEMBL4760911 | US11247971, Cmpd ID 410)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#C)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546971
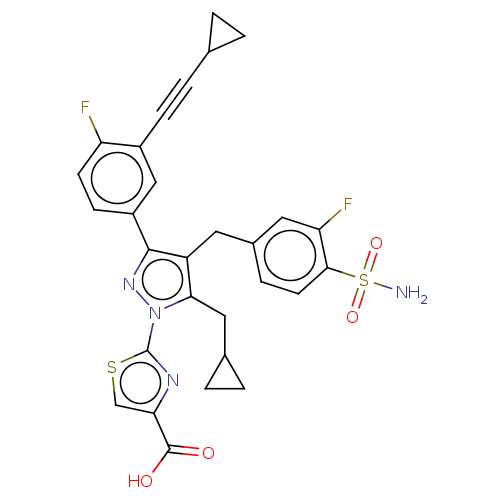
(CHEMBL4777867 | US11247971, Cmpd ID 262)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC2CC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546990

(CHEMBL4794789)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CCC2CCCO2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546999

(CHEMBL4786717)Show SMILES Cn1cc(cn1)C#Cc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 2419-22 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.111
BindingDB Entry DOI: 10.7270/Q2JW8DH8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 2419-22 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.111
BindingDB Entry DOI: 10.7270/Q2JW8DH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of ErbB4 |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of Btk |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
Activin receptor type-2B
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of ACTR-2B |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546976

(CHEMBL4751495)Show SMILES CC(C#Cc1cc(ccc1F)-c1nn(c(CC2CC2)c1Cc1ccc(c(F)c1)S(N)(=O)=O)-c1nc(cs1)C(O)=O)C1COC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM197160

(GNE-140 (6))Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@@](C1)(c1ccsc1)c1ccc(cc1)N1CCOCC1 |r,c:1| Show InChI InChI=1S/C25H23ClN2O3S2/c26-20-3-1-2-4-22(20)33-23-21(29)15-25(27-24(23)30,18-9-14-32-16-18)17-5-7-19(8-6-17)28-10-12-31-13-11-28/h1-9,14,16,29H,10-13,15H2,(H,27,30)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA using sodium pyruvate as substrate in presence of NAPDH |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |
Nuclear receptor coactivator 1
(Homo sapiens (Human)) | BDBM50349213

(CHEMBL1808264 | D3RKN_42)Show SMILES CC(C)c1nc(c(s1)-c1ccnc(Nc2ccc(nc2)N2CCOCC2)n1)-c1ccc(F)c(NS(=O)(=O)c2c(F)cccc2F)c1 Show InChI InChI=1S/C31H28F3N7O3S2/c1-18(2)30-39-27(19-6-8-21(32)25(16-19)40-46(42,43)29-22(33)4-3-5-23(29)34)28(45-30)24-10-11-35-31(38-24)37-20-7-9-26(36-17-20)41-12-14-44-15-13-41/h3-11,16-18,40H,12-15H2,1-2H3,(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Oncology CEDD
Curated by ChEMBL
| Assay Description
Inhibition of Src1 |
Bioorg Med Chem Lett 21: 4436-40 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.021
BindingDB Entry DOI: 10.7270/Q2Q240KZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data