 Found 255 hits with Last Name = 'wehle' and Initial = 's'
Found 255 hits with Last Name = 'wehle' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053435
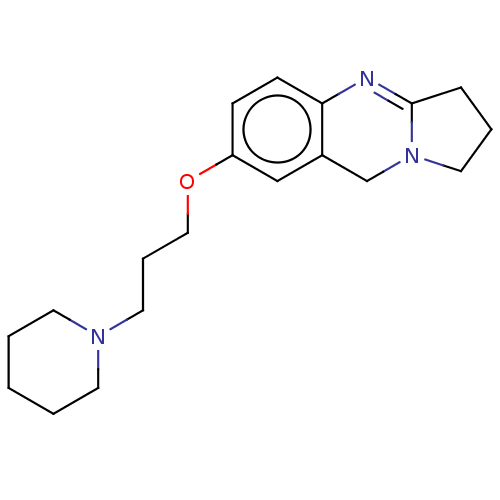
(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of human H3 receptor |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053433
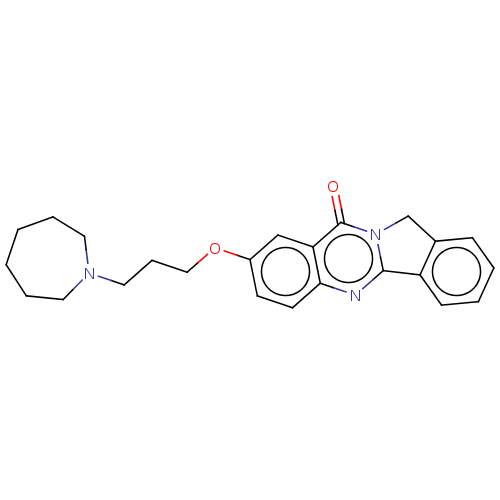
(CHEMBL3323037)Show SMILES O=c1n2Cc3ccccc3-c2nc2ccc(OCCCN3CCCCCC3)cc12 Show InChI InChI=1S/C24H27N3O2/c28-24-21-16-19(29-15-7-14-26-12-5-1-2-6-13-26)10-11-22(21)25-23-20-9-4-3-8-18(20)17-27(23)24/h3-4,8-11,16H,1-2,5-7,12-15,17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053433

(CHEMBL3323037)Show SMILES O=c1n2Cc3ccccc3-c2nc2ccc(OCCCN3CCCCCC3)cc12 Show InChI InChI=1S/C24H27N3O2/c28-24-21-16-19(29-15-7-14-26-12-5-1-2-6-13-26)10-11-22(21)25-23-20-9-4-3-8-18(20)17-27(23)24/h3-4,8-11,16H,1-2,5-7,12-15,17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053426
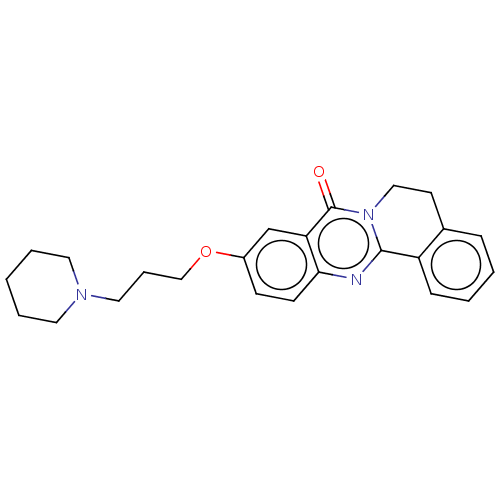
(CHEMBL3323042)Show SMILES O=c1n2CCc3ccccc3-c2nc2ccc(OCCCN3CCCCC3)cc12 Show InChI InChI=1S/C24H27N3O2/c28-24-21-17-19(29-16-6-14-26-12-4-1-5-13-26)9-10-22(21)25-23-20-8-3-2-7-18(20)11-15-27(23)24/h2-3,7-10,17H,1,4-6,11-16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053426

(CHEMBL3323042)Show SMILES O=c1n2CCc3ccccc3-c2nc2ccc(OCCCN3CCCCC3)cc12 Show InChI InChI=1S/C24H27N3O2/c28-24-21-17-19(29-16-6-14-26-12-4-1-5-13-26)9-10-22(21)25-23-20-8-3-2-7-18(20)11-15-27(23)24/h2-3,7-10,17H,1,4-6,11-16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053434
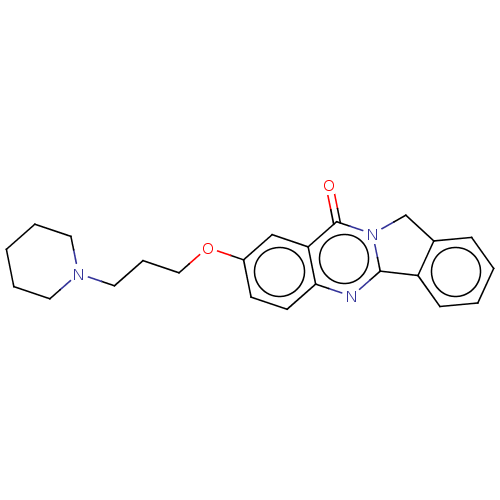
(CHEMBL3323036)Show InChI InChI=1S/C23H25N3O2/c27-23-20-15-18(28-14-6-13-25-11-4-1-5-12-25)9-10-21(20)24-22-19-8-3-2-7-17(19)16-26(22)23/h2-3,7-10,15H,1,4-6,11-14,16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053434

(CHEMBL3323036)Show InChI InChI=1S/C23H25N3O2/c27-23-20-15-18(28-14-6-13-25-11-4-1-5-12-25)9-10-21(20)24-22-19-8-3-2-7-17(19)16-26(22)23/h2-3,7-10,15H,1,4-6,11-14,16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
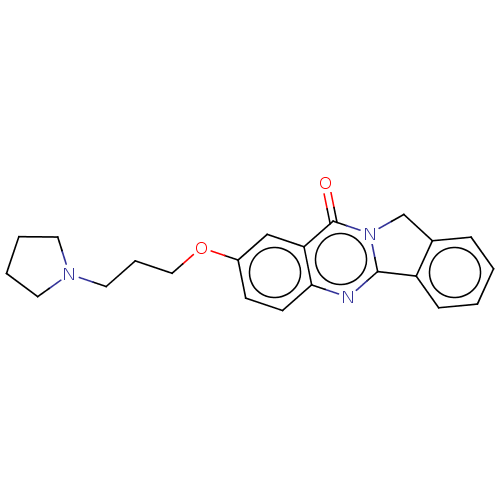
(Electrophorus electricus (Electric eel)) | BDBM50053429
(CHEMBL3323039)Show InChI InChI=1S/C22H25N3O2/c1-3-24(4-2)12-7-13-27-17-10-11-20-19(14-17)22(26)25-15-16-8-5-6-9-18(16)21(25)23-20/h5-6,8-11,14H,3-4,7,12-13,15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053429

(CHEMBL3323039)Show InChI InChI=1S/C22H25N3O2/c1-3-24(4-2)12-7-13-27-17-10-11-20-19(14-17)22(26)25-15-16-8-5-6-9-18(16)21(25)23-20/h5-6,8-11,14H,3-4,7,12-13,15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053425
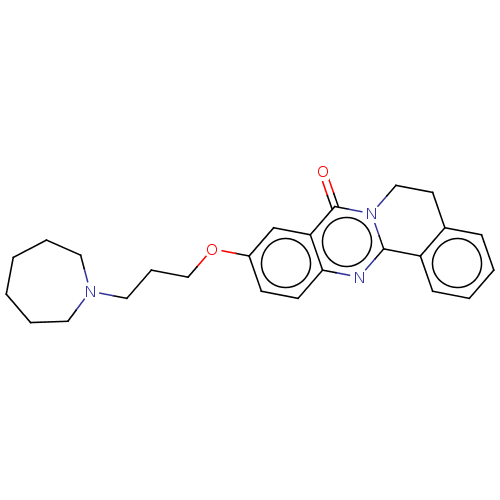
(CHEMBL3323020)Show SMILES O=c1n2CCc3ccccc3-c2nc2ccc(OCCCN3CCCCCC3)cc12 Show InChI InChI=1S/C25H29N3O2/c29-25-22-18-20(30-17-7-15-27-13-5-1-2-6-14-27)10-11-23(22)26-24-21-9-4-3-8-19(21)12-16-28(24)25/h3-4,8-11,18H,1-2,5-7,12-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50160078
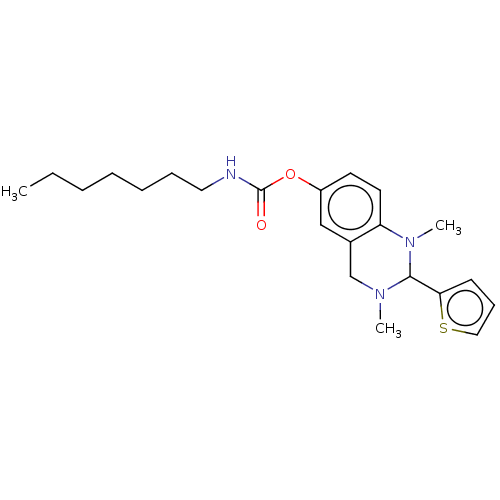
(CHEMBL3787613)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(C)Cc2c1)c1cccs1 Show InChI InChI=1S/C22H31N3O2S/c1-4-5-6-7-8-13-23-22(26)27-18-11-12-19-17(15-18)16-24(2)21(25(19)3)20-10-9-14-28-20/h9-12,14-15,21H,4-8,13,16H2,1-3H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of human butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured a... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053425

(CHEMBL3323020)Show SMILES O=c1n2CCc3ccccc3-c2nc2ccc(OCCCN3CCCCCC3)cc12 Show InChI InChI=1S/C25H29N3O2/c29-25-22-18-20(30-17-7-15-27-13-5-1-2-6-14-27)10-11-23(22)26-24-21-9-4-3-8-19(21)12-16-28(24)25/h3-4,8-11,18H,1-2,5-7,12-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160078

(CHEMBL3787613)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(C)Cc2c1)c1cccs1 Show InChI InChI=1S/C22H31N3O2S/c1-4-5-6-7-8-13-23-22(26)27-18-11-12-19-17(15-18)16-24(2)21(25(19)3)20-10-9-14-28-20/h9-12,14-15,21H,4-8,13,16H2,1-3H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053431
(CHEMBL3323038)Show InChI InChI=1S/C22H23N3O2/c26-22-19-14-17(27-13-5-12-24-10-3-4-11-24)8-9-20(19)23-21-18-7-2-1-6-16(18)15-25(21)22/h1-2,6-9,14H,3-5,10-13,15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053431

(CHEMBL3323038)Show InChI InChI=1S/C22H23N3O2/c26-22-19-14-17(27-13-5-12-24-10-3-4-11-24)8-9-20(19)23-21-18-7-2-1-6-16(18)15-25(21)22/h1-2,6-9,14H,3-5,10-13,15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160250
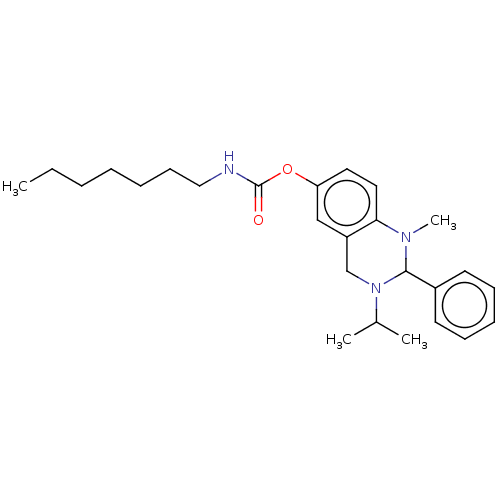
(CHEMBL3785472)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(Cc2c1)C(C)C)c1ccccc1 Show InChI InChI=1S/C26H37N3O2/c1-5-6-7-8-12-17-27-26(30)31-23-15-16-24-22(18-23)19-29(20(2)3)25(28(24)4)21-13-10-9-11-14-21/h9-11,13-16,18,20,25H,5-8,12,17,19H2,1-4H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160255

(CHEMBL3785189)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(C)Cc2c1)c1ccsc1 Show InChI InChI=1S/C22H31N3O2S/c1-4-5-6-7-8-12-23-22(26)27-19-9-10-20-18(14-19)15-24(2)21(25(20)3)17-11-13-28-16-17/h9-11,13-14,16,21H,4-8,12,15H2,1-3H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160077
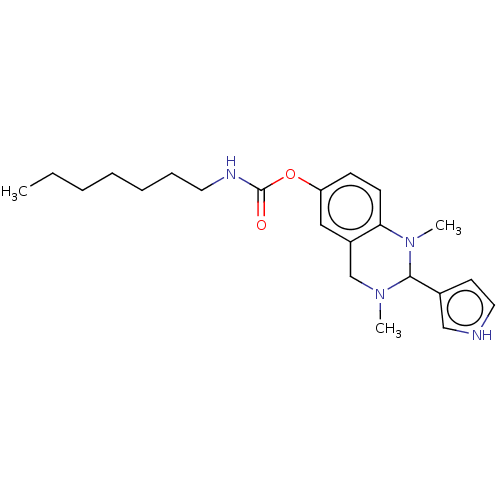
(CHEMBL3785861)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(C)Cc2c1)c1cc[nH]c1 Show InChI InChI=1S/C22H32N4O2/c1-4-5-6-7-8-12-24-22(27)28-19-9-10-20-18(14-19)16-25(2)21(26(20)3)17-11-13-23-15-17/h9-11,13-15,21,23H,4-8,12,16H2,1-3H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053439
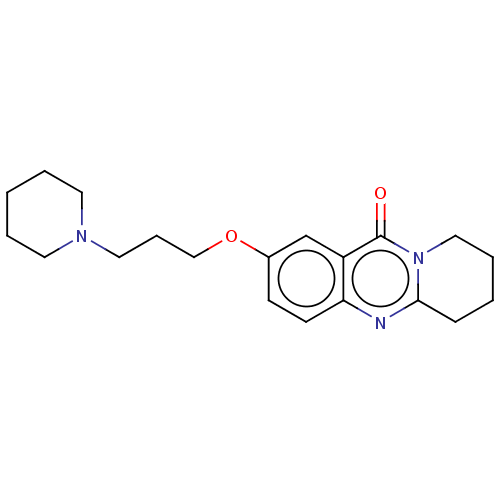
(CHEMBL3323059)Show InChI InChI=1S/C20H27N3O2/c24-20-17-15-16(25-14-6-12-22-10-3-1-4-11-22)8-9-18(17)21-19-7-2-5-13-23(19)20/h8-9,15H,1-7,10-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053439

(CHEMBL3323059)Show InChI InChI=1S/C20H27N3O2/c24-20-17-15-16(25-14-6-12-22-10-3-1-4-11-22)8-9-18(17)21-19-7-2-5-13-23(19)20/h8-9,15H,1-7,10-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160247
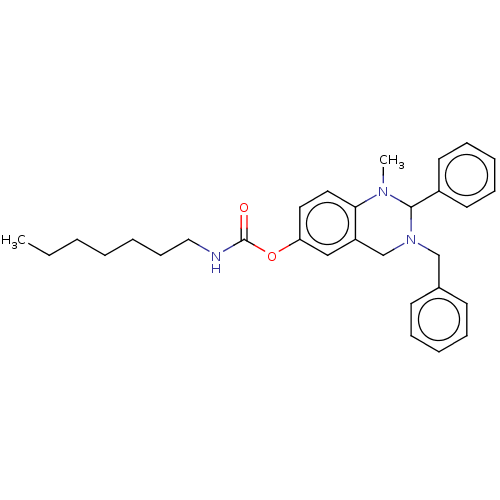
(CHEMBL3786119)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(Cc3ccccc3)Cc2c1)c1ccccc1 Show InChI InChI=1S/C18H21NOS/c1-14-6-5-9-16(12-14)21-18(15-7-3-2-4-8-15)17-13-19-10-11-20-17/h2-9,12,17-19H,10-11,13H2,1H3/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
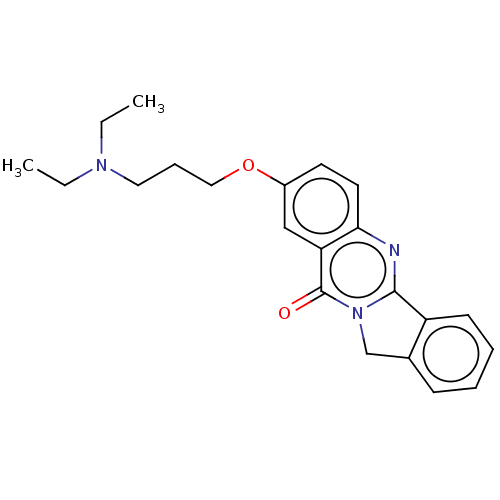
(Homo sapiens (Human)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053453

(CHEMBL3323066)Show InChI InChI=1S/C22H31N3O2/c26-22-19-17-18(27-16-8-14-24-12-5-1-2-6-13-24)10-11-20(19)23-21-9-4-3-7-15-25(21)22/h10-11,17H,1-9,12-16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053453

(CHEMBL3323066)Show InChI InChI=1S/C22H31N3O2/c26-22-19-17-18(27-16-8-14-24-12-5-1-2-6-13-24)10-11-20(19)23-21-9-4-3-7-15-25(21)22/h10-11,17H,1-9,12-16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160249
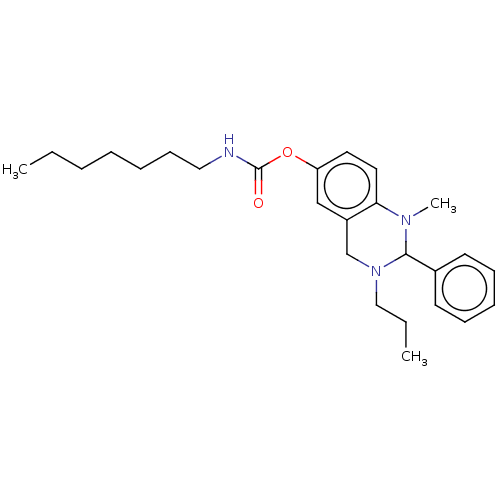
(CHEMBL3785181)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(CCC)Cc2c1)c1ccccc1 Show InChI InChI=1S/C17H19NOS/c1-3-7-14(8-4-1)17(16-13-18-11-12-19-16)20-15-9-5-2-6-10-15/h1-10,16-18H,11-13H2/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053437
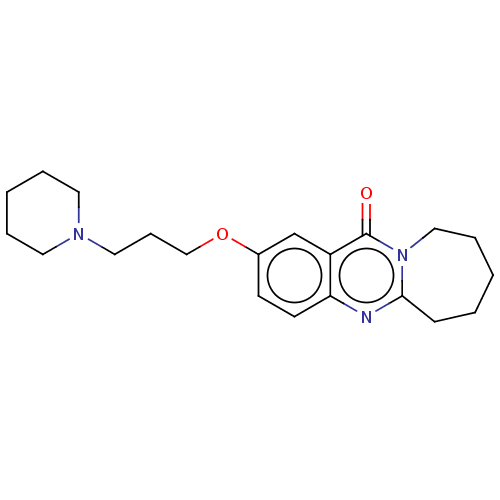
(CHEMBL3323065)Show InChI InChI=1S/C21H29N3O2/c25-21-18-16-17(26-15-7-13-23-11-4-2-5-12-23)9-10-19(18)22-20-8-3-1-6-14-24(20)21/h9-10,16H,1-8,11-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053438
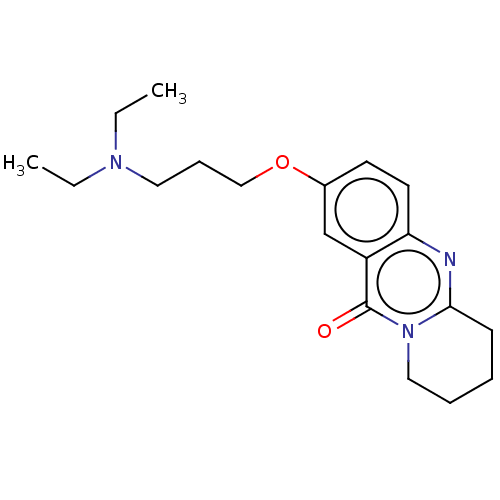
(CHEMBL3323062)Show InChI InChI=1S/C19H27N3O2/c1-3-21(4-2)11-7-13-24-15-9-10-17-16(14-15)19(23)22-12-6-5-8-18(22)20-17/h9-10,14H,3-8,11-13H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053437

(CHEMBL3323065)Show InChI InChI=1S/C21H29N3O2/c25-21-18-16-17(26-15-7-13-23-11-4-2-5-12-23)9-10-19(18)22-20-8-3-1-6-14-24(20)21/h9-10,16H,1-8,11-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053438

(CHEMBL3323062)Show InChI InChI=1S/C19H27N3O2/c1-3-21(4-2)11-7-13-24-15-9-10-17-16(14-15)19(23)22-12-6-5-8-18(22)20-17/h9-10,14H,3-8,11-13H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50160076
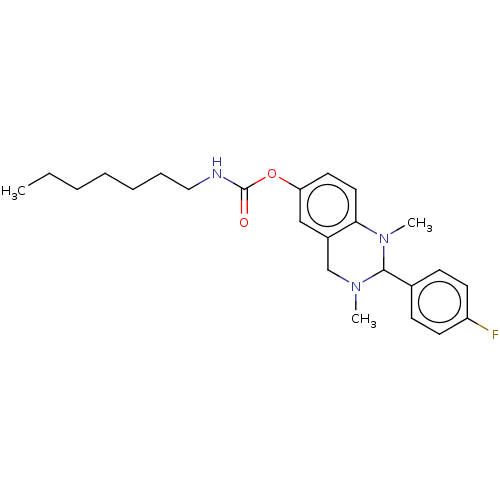
(CHEMBL3787116)Show SMILES CCCCCCCNC(=O)Oc1ccc2N(C)C(N(C)Cc2c1)c1ccc(F)cc1 Show InChI InChI=1S/C24H32FN3O2/c1-4-5-6-7-8-15-26-24(29)30-21-13-14-22-19(16-21)17-27(2)23(28(22)3)18-9-11-20(25)12-10-18/h9-14,16,23H,4-8,15,17H2,1-3H3,(H,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine serum butyrylcholinesterase using butyrylcholine iodide as substrate preincubated for 30 mins followed by substrate addition mea... |
J Med Chem 59: 2067-82 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01674
BindingDB Entry DOI: 10.7270/Q25H7J4D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053422

(CHEMBL3323043)Show InChI InChI=1S/C23H27N3O2/c1-3-25(4-2)13-7-15-28-18-10-11-21-20(16-18)23(27)26-14-12-17-8-5-6-9-19(17)22(26)24-21/h5-6,8-11,16H,3-4,7,12-15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053422

(CHEMBL3323043)Show InChI InChI=1S/C23H27N3O2/c1-3-25(4-2)13-7-15-28-18-10-11-21-20(16-18)23(27)26-14-12-17-8-5-6-9-19(17)22(26)24-21/h5-6,8-11,16H,3-4,7,12-15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053445
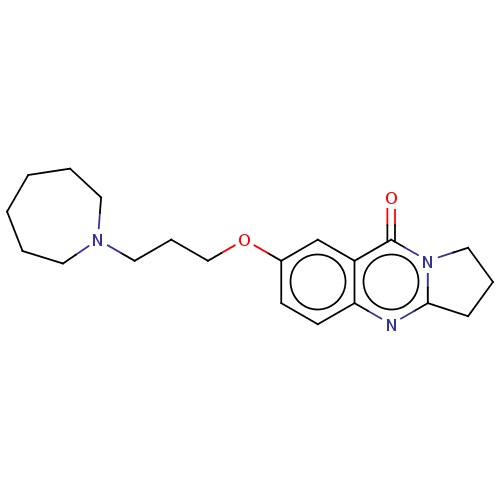
(CHEMBL3323054)Show InChI InChI=1S/C20H27N3O2/c24-20-17-15-16(8-9-18(17)21-19-7-5-13-23(19)20)25-14-6-12-22-10-3-1-2-4-11-22/h8-9,15H,1-7,10-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053445

(CHEMBL3323054)Show InChI InChI=1S/C20H27N3O2/c24-20-17-15-16(8-9-18(17)21-19-7-5-13-23(19)20)25-14-6-12-22-10-3-1-2-4-11-22/h8-9,15H,1-7,10-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053446
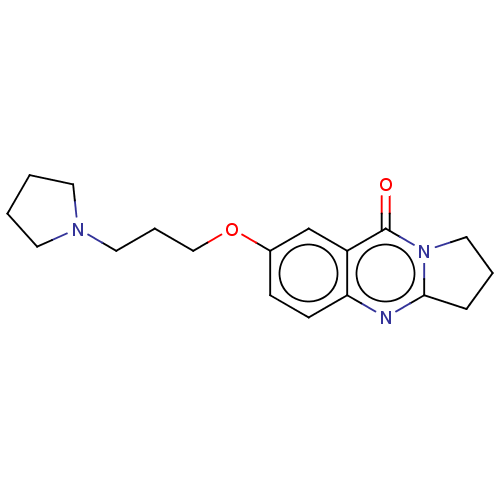
(CHEMBL3323055)Show InChI InChI=1S/C18H23N3O2/c22-18-15-13-14(23-12-4-10-20-8-1-2-9-20)6-7-16(15)19-17-5-3-11-21(17)18/h6-7,13H,1-5,8-12H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053446

(CHEMBL3323055)Show InChI InChI=1S/C18H23N3O2/c22-18-15-13-14(23-12-4-10-20-8-1-2-9-20)6-7-16(15)19-17-5-3-11-21(17)18/h6-7,13H,1-5,8-12H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053436
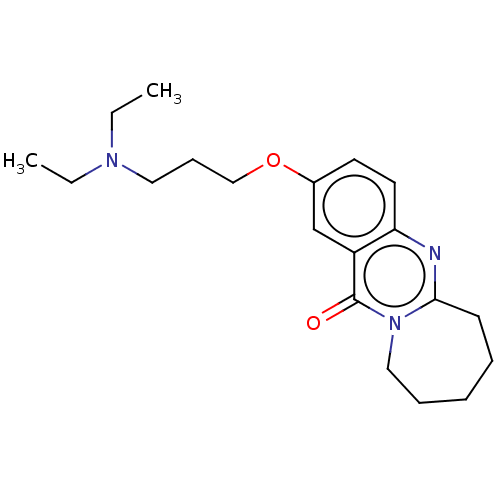
(CHEMBL3323068)Show InChI InChI=1S/C20H29N3O2/c1-3-22(4-2)12-8-14-25-16-10-11-18-17(15-16)20(24)23-13-7-5-6-9-19(23)21-18/h10-11,15H,3-9,12-14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053436

(CHEMBL3323068)Show InChI InChI=1S/C20H29N3O2/c1-3-22(4-2)12-8-14-25-16-10-11-18-17(15-16)20(24)23-13-7-5-6-9-19(23)21-18/h10-11,15H,3-9,12-14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053569
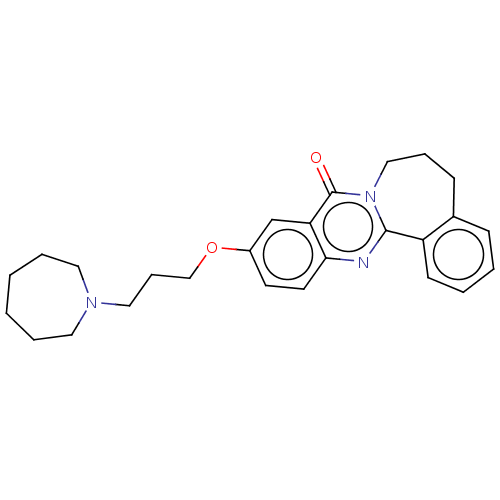
(CHEMBL3323047)Show SMILES O=c1n2CCCc3ccccc3-c2nc2ccc(OCCCN3CCCCCC3)cc12 Show InChI InChI=1S/C26H31N3O2/c30-26-23-19-21(31-18-8-16-28-14-5-1-2-6-15-28)12-13-24(23)27-25-22-11-4-3-9-20(22)10-7-17-29(25)26/h3-4,9,11-13,19H,1-2,5-8,10,14-18H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053569

(CHEMBL3323047)Show SMILES O=c1n2CCCc3ccccc3-c2nc2ccc(OCCCN3CCCCCC3)cc12 Show InChI InChI=1S/C26H31N3O2/c30-26-23-19-21(31-18-8-16-28-14-5-1-2-6-15-28)12-13-24(23)27-25-22-11-4-3-9-20(22)10-7-17-29(25)26/h3-4,9,11-13,19H,1-2,5-8,10,14-18H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053419
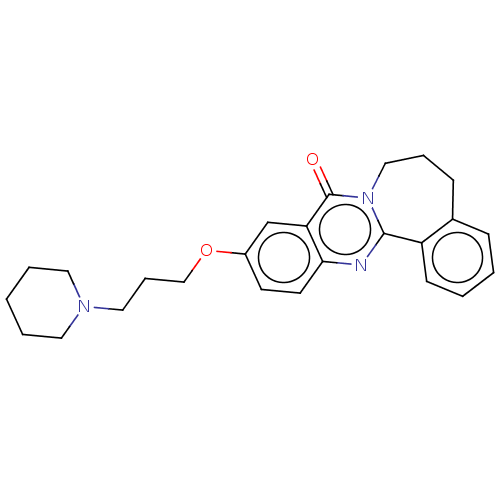
(CHEMBL3323046)Show SMILES O=c1n2CCCc3ccccc3-c2nc2ccc(OCCCN3CCCCC3)cc12 Show InChI InChI=1S/C25H29N3O2/c29-25-22-18-20(30-17-7-15-27-13-4-1-5-14-27)11-12-23(22)26-24-21-10-3-2-8-19(21)9-6-16-28(24)25/h2-3,8,10-12,18H,1,4-7,9,13-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053419

(CHEMBL3323046)Show SMILES O=c1n2CCCc3ccccc3-c2nc2ccc(OCCCN3CCCCC3)cc12 Show InChI InChI=1S/C25H29N3O2/c29-25-22-18-20(30-17-7-15-27-13-4-1-5-14-27)11-12-23(22)26-24-21-10-3-2-8-19(21)9-6-16-28(24)25/h2-3,8,10-12,18H,1,4-7,9,13-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053449
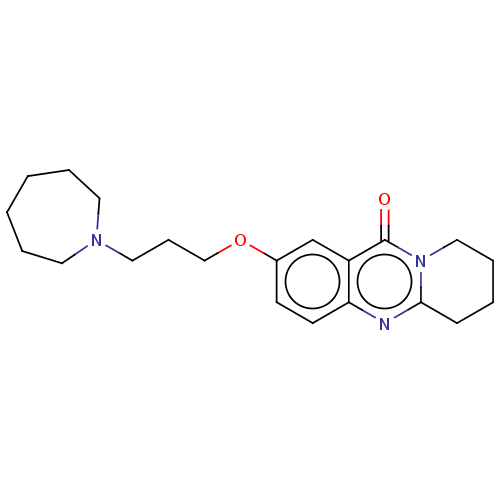
(CHEMBL3323060)Show InChI InChI=1S/C21H29N3O2/c25-21-18-16-17(26-15-7-13-23-11-4-1-2-5-12-23)9-10-19(18)22-20-8-3-6-14-24(20)21/h9-10,16H,1-8,11-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053449

(CHEMBL3323060)Show InChI InChI=1S/C21H29N3O2/c25-21-18-16-17(26-15-7-13-23-11-4-1-2-5-12-23)9-10-19(18)22-20-8-3-6-14-24(20)21/h9-10,16H,1-8,11-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data