 Found 43 hits with Last Name = 'weissman' and Initial = 'ba'
Found 43 hits with Last Name = 'weissman' and Initial = 'ba' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50370403

(CHEMBL177829)Show SMILES O=C1NC(C(=O)N1CCCCCN1CCC(CC1)c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C31H35N3O2/c35-29-31(27-15-7-2-8-16-27,28-17-9-3-10-18-28)32-30(36)34(29)22-12-4-11-21-33-23-19-26(20-24-33)25-13-5-1-6-14-25/h1-3,5-10,13-18,26H,4,11-12,19-24H2,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to Sigma opioid receptor type 1 in guinea pig brain homogenate with 0.5 nM of [3H](+)-PENT as radioligand |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50370403

(CHEMBL177829)Show SMILES O=C1NC(C(=O)N1CCCCCN1CCC(CC1)c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C31H35N3O2/c35-29-31(27-15-7-2-8-16-27,28-17-9-3-10-18-28)32-30(36)34(29)22-12-4-11-21-33-23-19-26(20-24-33)25-13-5-1-6-14-25/h1-3,5-10,13-18,26H,4,11-12,19-24H2,(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to Sigma opioid receptor type 2 in guinea pig brain homogenate with 4 nM of [3H](+)-DTG as radioligand |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
3-dehydroquinate synthase
(Escherichia coli (strain K12)) | BDBM50028881
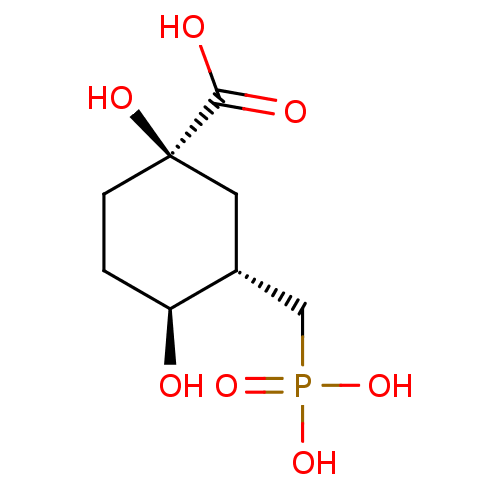
((1R,3S,4S)-1,4-Dihydroxy-3-phosphonomethyl-cyclohe...)Show SMILES O[C@H]1CC[C@@](O)(C[C@@H]1CP(O)(O)=O)C(O)=O Show InChI InChI=1S/C8H15O7P/c9-6-1-2-8(12,7(10)11)3-5(6)4-16(13,14)15/h5-6,9,12H,1-4H2,(H,10,11)(H2,13,14,15)/t5-,6+,8-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory constant against 3-dehydroquinate synthase |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50409932

(CHEMBL178537)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)C(C)(C)C=C Show InChI InChI=1S/C27H39NO4/c1-7-26(3,4)22(18-17-20-14-10-9-11-15-20)32-25(31)21-16-12-13-19-28(21)24(30)23(29)27(5,6)8-2/h7,9-11,14-15,21-22H,1,8,12-13,16-19H2,2-6H3/t21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370391
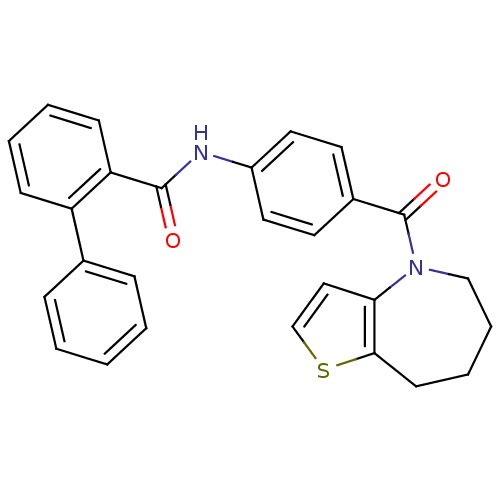
(CHEMBL175813)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2sccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H24N2O2S/c31-27(24-11-5-4-10-23(24)20-8-2-1-3-9-20)29-22-15-13-21(14-16-22)28(32)30-18-7-6-12-26-25(30)17-19-33-26/h1-5,8-11,13-17,19H,6-7,12,18H2,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370408
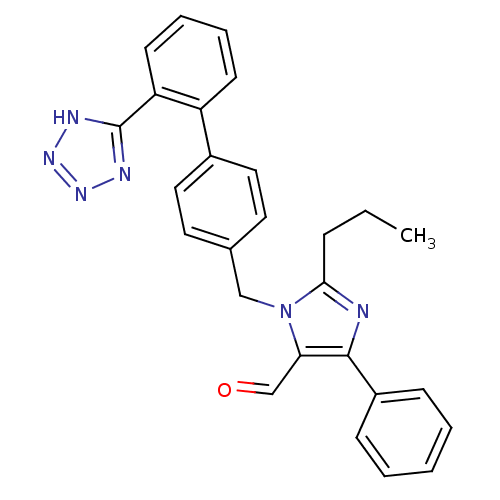
(CHEMBL177435)Show SMILES CCCc1nc(c(C=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1ccccc1 Show InChI InChI=1S/C27H24N6O/c1-2-8-25-28-26(21-9-4-3-5-10-21)24(18-34)33(25)17-19-13-15-20(16-14-19)22-11-6-7-12-23(22)27-29-31-32-30-27/h3-7,9-16,18H,2,8,17H2,1H3,(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against angiotensin II AT-2 receptor |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370394
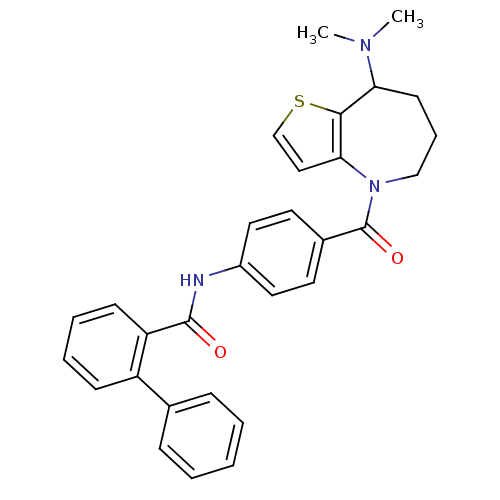
(CHEMBL368791)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccsc12 Show InChI InChI=1S/C30H29N3O2S/c1-32(2)26-13-8-19-33(27-18-20-36-28(26)27)30(35)22-14-16-23(17-15-22)31-29(34)25-12-7-6-11-24(25)21-9-4-3-5-10-21/h3-7,9-12,14-18,20,26H,8,13,19H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370404
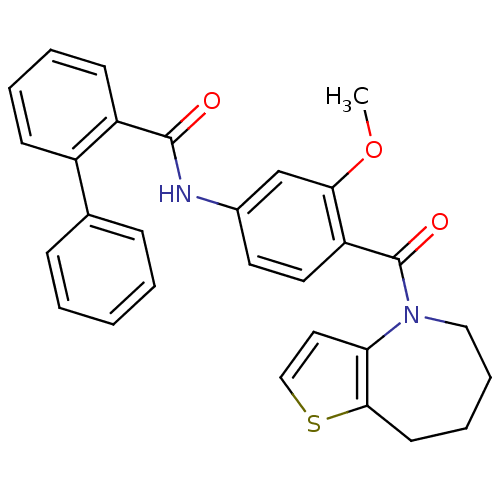
(CHEMBL175930)Show SMILES COc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C29H26N2O3S/c1-34-26-19-21(30-28(32)23-12-6-5-11-22(23)20-9-3-2-4-10-20)14-15-24(26)29(33)31-17-8-7-13-27-25(31)16-18-35-27/h2-6,9-12,14-16,18-19H,7-8,13,17H2,1H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370394

(CHEMBL368791)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccsc12 Show InChI InChI=1S/C30H29N3O2S/c1-32(2)26-13-8-19-33(27-18-20-36-28(26)27)30(35)22-14-16-23(17-15-22)31-29(34)25-12-7-6-11-24(25)21-9-4-3-5-10-21/h3-7,9-12,14-18,20,26H,8,13,19H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370404

(CHEMBL175930)Show SMILES COc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C29H26N2O3S/c1-34-26-19-21(30-28(32)23-12-6-5-11-22(23)20-9-3-2-4-10-20)14-15-24(26)29(33)31-17-8-7-13-27-25(31)16-18-35-27/h2-6,9-12,14-16,18-19H,7-8,13,17H2,1H3,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028866
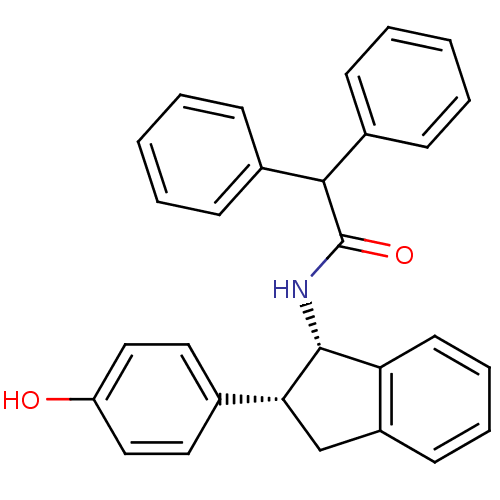
(CHEMBL297271 | N-[(1S,2R)-2-(4-Hydroxy-phenyl)-ind...)Show SMILES Oc1ccc(cc1)[C@H]1Cc2ccccc2[C@H]1NC(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H25NO2/c31-24-17-15-20(16-18-24)26-19-23-13-7-8-14-25(23)28(26)30-29(32)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-18,26-28,31H,19H2,(H,30,32)/t26-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370397
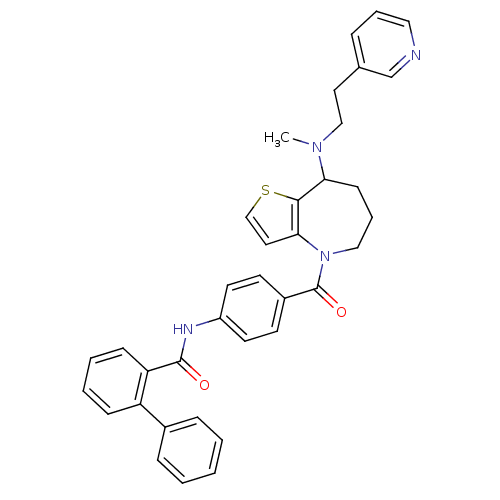
(CHEMBL177556)Show SMILES CN(CCc1cccnc1)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccsc12 Show InChI InChI=1S/C36H34N4O2S/c1-39(23-19-26-9-7-21-37-25-26)32-14-8-22-40(33-20-24-43-34(32)33)36(42)28-15-17-29(18-16-28)38-35(41)31-13-6-5-12-30(31)27-10-3-2-4-11-27/h2-7,9-13,15-18,20-21,24-25,32H,8,14,19,22-23H2,1H3,(H,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370397

(CHEMBL177556)Show SMILES CN(CCc1cccnc1)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccsc12 Show InChI InChI=1S/C36H34N4O2S/c1-39(23-19-26-9-7-21-37-25-26)32-14-8-22-40(33-20-24-43-34(32)33)36(42)28-15-17-29(18-16-28)38-35(41)31-13-6-5-12-30(31)27-10-3-2-4-11-27/h2-7,9-13,15-18,20-21,24-25,32H,8,14,19,22-23H2,1H3,(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370400
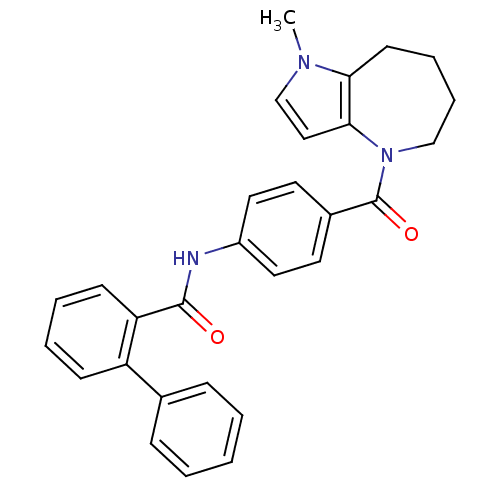
(CHEMBL176264)Show SMILES Cn1ccc2N(CCCCc12)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C29H27N3O2/c1-31-20-18-27-26(31)13-7-8-19-32(27)29(34)22-14-16-23(17-15-22)30-28(33)25-12-6-5-11-24(25)21-9-3-2-4-10-21/h2-6,9-12,14-18,20H,7-8,13,19H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370405
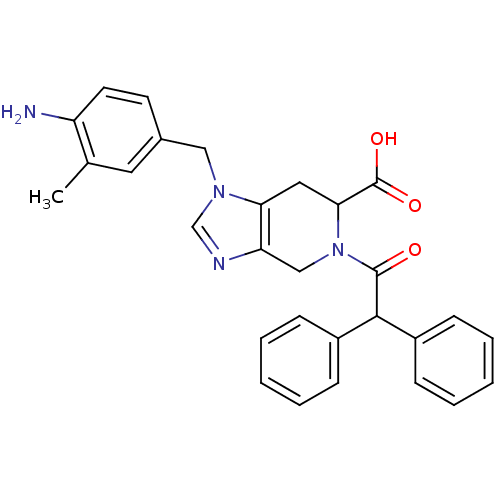
(CHEMBL535139 | PD-123177)Show SMILES Cc1cc(Cn2cnc3CN(C(Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)ccc1N Show InChI InChI=1S/C29H28N4O3/c1-19-14-20(12-13-23(19)30)16-32-18-31-24-17-33(26(29(35)36)15-25(24)32)28(34)27(21-8-4-2-5-9-21)22-10-6-3-7-11-22/h2-14,18,26-27H,15-17,30H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against angiotensin II AT-2 receptor |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50226247
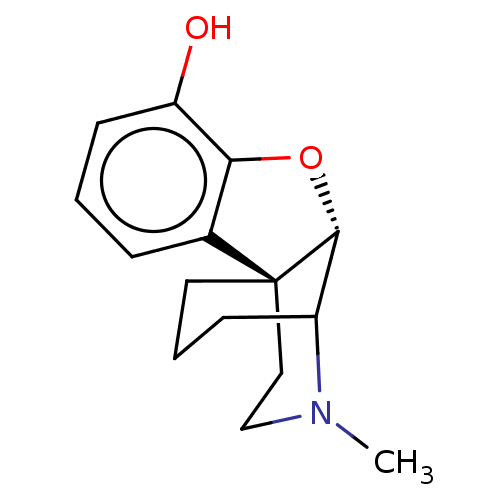
(CHEMBL423449)Show SMILES [H][C@]12Oc3c(cccc3O)[C@@]11CCCC2N(C)CC1 |TLB:16:15:1:11.13.12| Show InChI InChI=1S/C15H19NO2/c1-16-9-8-15-7-3-5-11(16)14(15)18-13-10(15)4-2-6-12(13)17/h2,4,6,11,14,17H,3,5,7-9H2,1H3/t11?,14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards opioid receptor |
J Med Chem 29: 748-51 (1986)
BindingDB Entry DOI: 10.7270/Q2PG1TX2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370392

(CHEMBL369712)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccsc12 Show InChI InChI=1S/C25H27N3O2S/c1-17-7-4-5-8-20(17)24(29)26-19-12-10-18(11-13-19)25(30)28-15-6-9-21(27(2)3)23-22(28)14-16-31-23/h4-5,7-8,10-14,16,21H,6,9,15H2,1-3H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370402
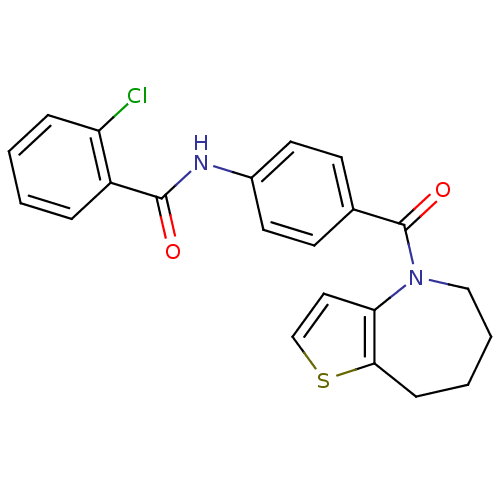
(CHEMBL441498)Show SMILES Clc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C22H19ClN2O2S/c23-18-6-2-1-5-17(18)21(26)24-16-10-8-15(9-11-16)22(27)25-13-4-3-7-20-19(25)12-14-28-20/h1-2,5-6,8-12,14H,3-4,7,13H2,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370398
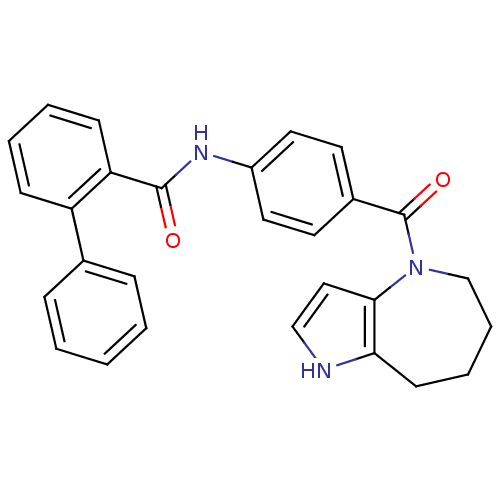
(CHEMBL175211)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2[nH]ccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H25N3O2/c32-27(24-11-5-4-10-23(24)20-8-2-1-3-9-20)30-22-15-13-21(14-16-22)28(33)31-19-7-6-12-25-26(31)17-18-29-25/h1-5,8-11,13-18,29H,6-7,12,19H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370395
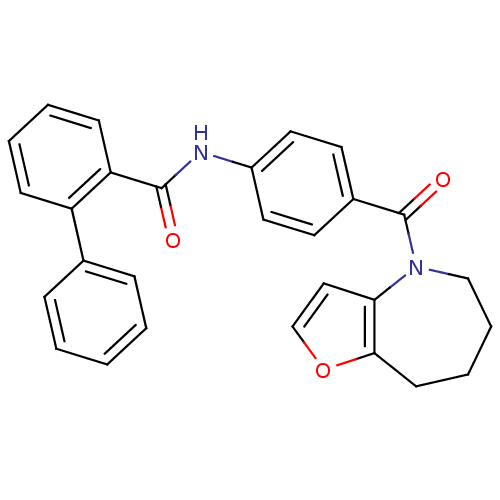
(CHEMBL175411)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2occc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H24N2O3/c31-27(24-11-5-4-10-23(24)20-8-2-1-3-9-20)29-22-15-13-21(14-16-22)28(32)30-18-7-6-12-26-25(30)17-19-33-26/h1-5,8-11,13-17,19H,6-7,12,18H2,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370391

(CHEMBL175813)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2sccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H24N2O2S/c31-27(24-11-5-4-10-23(24)20-8-2-1-3-9-20)29-22-15-13-21(14-16-22)28(32)30-18-7-6-12-26-25(30)17-19-33-26/h1-5,8-11,13-17,19H,6-7,12,18H2,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370400

(CHEMBL176264)Show SMILES Cn1ccc2N(CCCCc12)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C29H27N3O2/c1-31-20-18-27-26(31)13-7-8-19-32(27)29(34)22-14-16-23(17-15-22)30-28(33)25-12-6-5-11-24(25)21-9-3-2-4-10-21/h2-6,9-12,14-18,20H,7-8,13,19H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028868
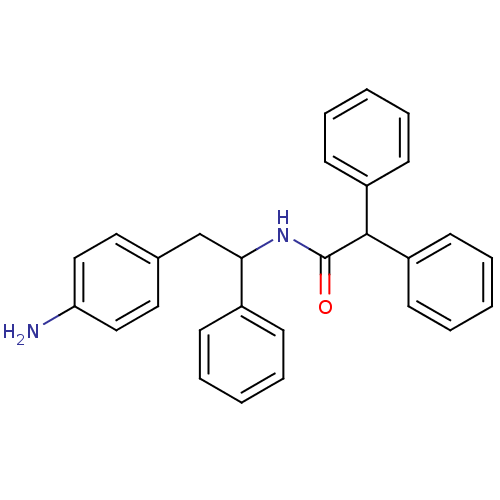
(CHEMBL41233 | N-[2-(4-Amino-phenyl)-1-phenyl-ethyl...)Show SMILES Nc1ccc(CC(NC(=O)C(c2ccccc2)c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C28H26N2O/c29-25-18-16-21(17-19-25)20-26(22-10-4-1-5-11-22)30-28(31)27(23-12-6-2-7-13-23)24-14-8-3-9-15-24/h1-19,26-27H,20,29H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50030448
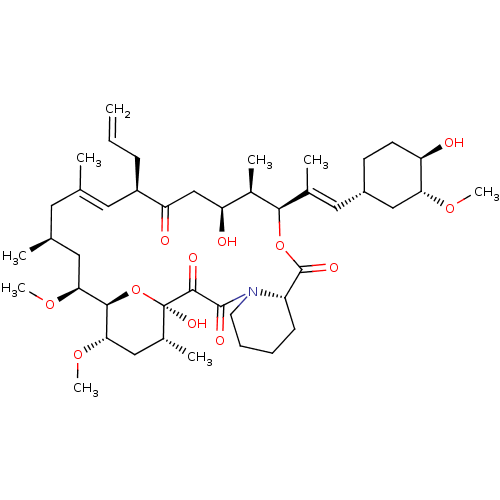
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinant FK506 binding protein 12 to determine the FKBP binding property of the compound |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50226268
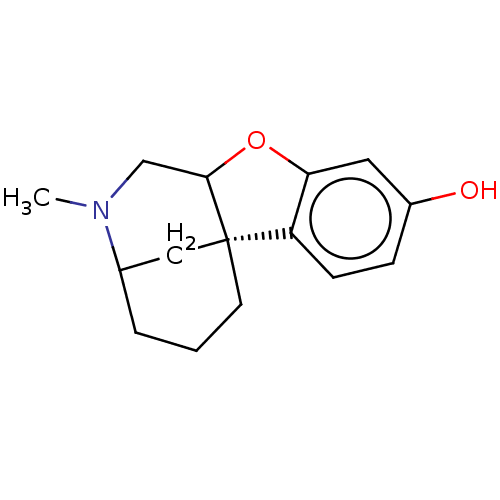
(CHEMBL348954)Show InChI InChI=1S/C15H19NO2/c1-16-9-14-15(6-2-3-10(16)8-15)12-5-4-11(17)7-13(12)18-14/h4-5,7,10,14,17H,2-3,6,8-9H2,1H3/t10?,14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards opioid receptor |
J Med Chem 29: 748-51 (1986)
BindingDB Entry DOI: 10.7270/Q2PG1TX2 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028880
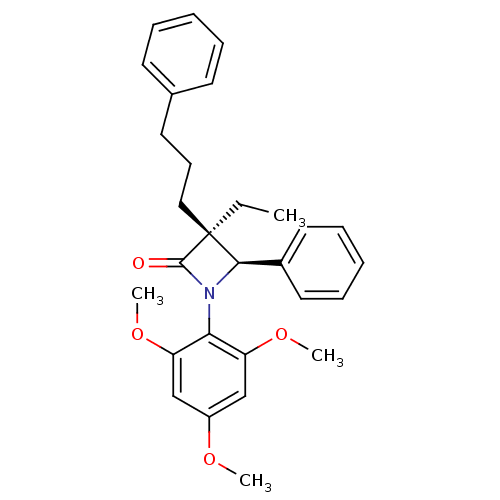
((3S,4R)-3-Ethyl-4-phenyl-3-(3-phenyl-propyl)-1-(2,...)Show SMILES CC[C@]1(CCCc2ccccc2)[C@H](N(C1=O)c1c(OC)cc(OC)cc1OC)c1ccccc1 Show InChI InChI=1S/C29H33NO4/c1-5-29(18-12-15-21-13-8-6-9-14-21)27(22-16-10-7-11-17-22)30(28(29)31)26-24(33-3)19-23(32-2)20-25(26)34-4/h6-11,13-14,16-17,19-20,27H,5,12,15,18H2,1-4H3/t27-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50226243
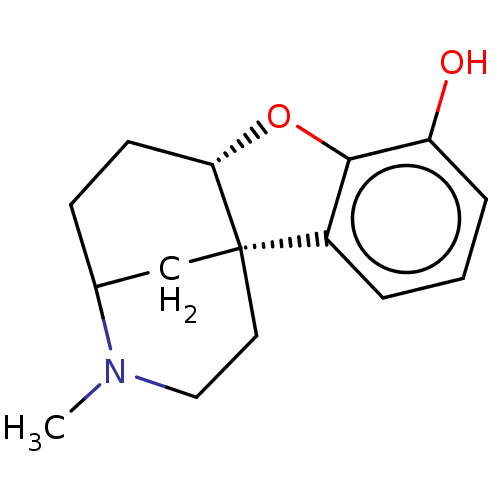
(CHEMBL353357)Show SMILES [H][C@]12CCC3C[C@@]1(CCN3C)c1cccc(O)c1O2 |THB:10:9:5:1.2.3| Show InChI InChI=1S/C15H19NO2/c1-16-8-7-15-9-10(16)5-6-13(15)18-14-11(15)3-2-4-12(14)17/h2-4,10,13,17H,5-9H2,1H3/t10?,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards opioid receptor |
J Med Chem 29: 748-51 (1986)
BindingDB Entry DOI: 10.7270/Q2PG1TX2 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005944
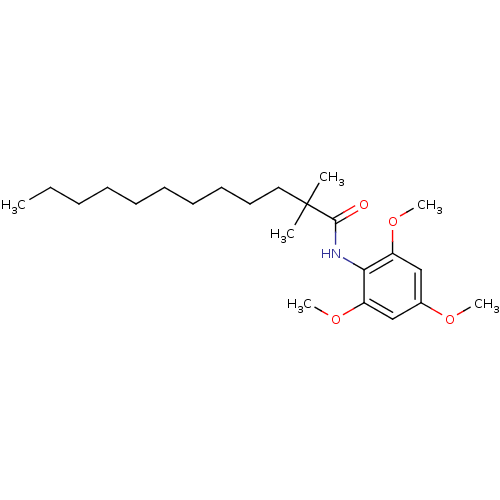
(2,2-Dimethyl-dodecanoic acid (2,4,6-trimethoxy-phe...)Show InChI InChI=1S/C23H39NO4/c1-7-8-9-10-11-12-13-14-15-23(2,3)22(25)24-21-19(27-5)16-18(26-4)17-20(21)28-6/h16-17H,7-15H2,1-6H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50370399
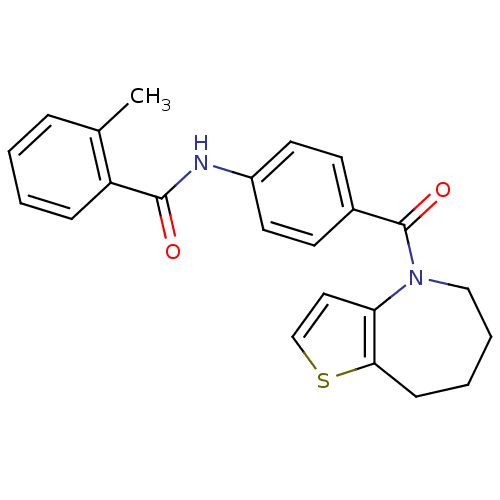
(CHEMBL360272)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C23H22N2O2S/c1-16-6-2-3-7-19(16)22(26)24-18-11-9-17(10-12-18)23(27)25-14-5-4-8-21-20(25)13-15-28-21/h2-3,6-7,9-13,15H,4-5,8,14H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028888
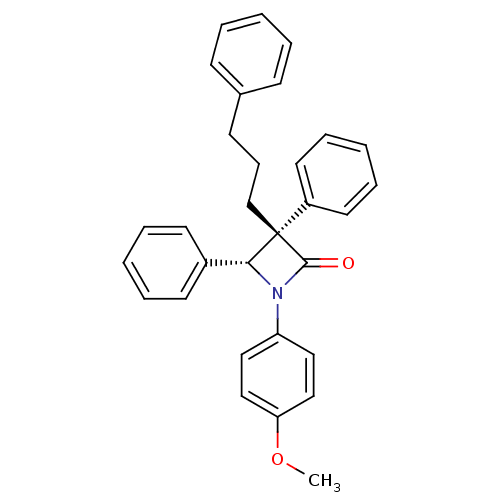
((3S,4R)-1-(4-Methoxy-phenyl)-3,4-diphenyl-3-(3-phe...)Show SMILES COc1ccc(cc1)N1[C@H](c2ccccc2)[C@@](CCCc2ccccc2)(C1=O)c1ccccc1 Show InChI InChI=1S/C31H29NO2/c1-34-28-21-19-27(20-22-28)32-29(25-15-7-3-8-16-25)31(30(32)33,26-17-9-4-10-18-26)23-11-14-24-12-5-2-6-13-24/h2-10,12-13,15-22,29H,11,14,23H2,1H3/t29-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028877

((3S,4R)-1-(4-Methoxy-phenyl)-3,4-diphenyl-3-phenyl...)Show SMILES COc1ccc(cc1)N1[C@H](c2ccccc2)[C@@](C(=O)Cc2ccccc2)(C1=O)c1ccccc1 Show InChI InChI=1S/C30H25NO3/c1-34-26-19-17-25(18-20-26)31-28(23-13-7-3-8-14-23)30(29(31)33,24-15-9-4-10-16-24)27(32)21-22-11-5-2-6-12-22/h2-20,28H,21H2,1H3/t28-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50370396
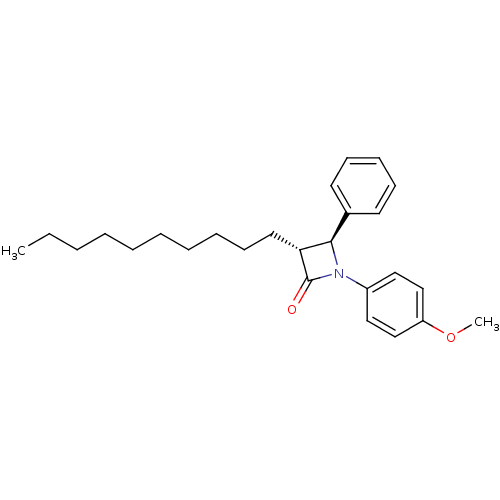
(CHEMBL178591)Show SMILES CCCCCCCCCC[C@@H]1[C@H](N(C1=O)c1ccc(OC)cc1)c1ccccc1 Show InChI InChI=1S/C26H35NO2/c1-3-4-5-6-7-8-9-13-16-24-25(21-14-11-10-12-15-21)27(26(24)28)22-17-19-23(29-2)20-18-22/h10-12,14-15,17-20,24-25H,3-9,13,16H2,1-2H3/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50370409
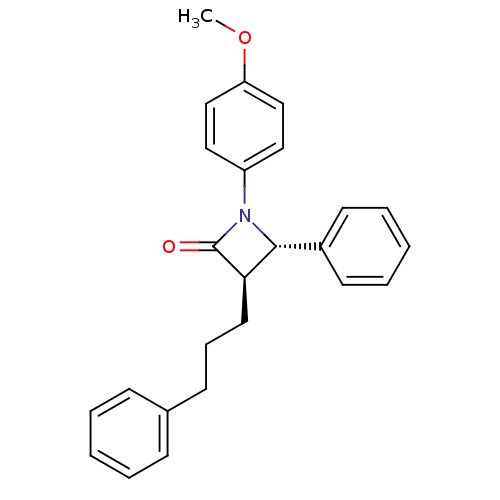
(CHEMBL332150)Show SMILES COc1ccc(cc1)N1[C@@H]([C@@H](CCCc2ccccc2)C1=O)c1ccccc1 Show InChI InChI=1S/C25H25NO2/c1-28-22-17-15-21(16-18-22)26-24(20-12-6-3-7-13-20)23(25(26)27)14-8-11-19-9-4-2-5-10-19/h2-7,9-10,12-13,15-18,23-24H,8,11,14H2,1H3/t23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370402

(CHEMBL441498)Show SMILES Clc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C22H19ClN2O2S/c23-18-6-2-1-5-17(18)21(26)24-16-10-8-15(9-11-16)22(27)25-13-4-3-7-20-19(25)12-14-28-20/h1-2,5-6,8-12,14H,3-4,7,13H2,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370399

(CHEMBL360272)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C23H22N2O2S/c1-16-6-2-3-7-19(16)22(26)24-18-11-9-17(10-12-18)23(27)25-14-5-4-8-21-20(25)13-15-28-21/h2-3,6-7,9-13,15H,4-5,8,14H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370398

(CHEMBL175211)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2[nH]ccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H25N3O2/c32-27(24-11-5-4-10-23(24)20-8-2-1-3-9-20)30-22-15-13-21(14-16-22)28(33)31-19-7-6-12-25-26(31)17-18-29-25/h1-5,8-11,13-18,29H,6-7,12,19H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50370410
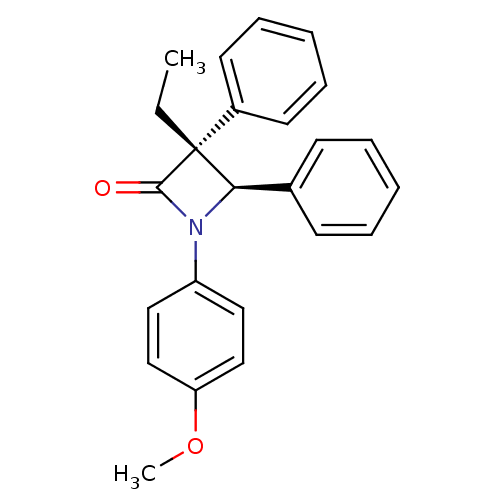
(CHEMBL369660)Show SMILES CC[C@]1([C@H](N(C1=O)c1ccc(OC)cc1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H23NO2/c1-3-24(19-12-8-5-9-13-19)22(18-10-6-4-7-11-18)25(23(24)26)20-14-16-21(27-2)17-15-20/h4-17,22H,3H2,1-2H3/t22-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50370395

(CHEMBL175411)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2occc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H24N2O3/c31-27(24-11-5-4-10-23(24)20-8-2-1-3-9-20)29-22-15-13-21(14-16-22)28(32)30-18-7-6-12-26-25(30)17-19-33-26/h1-5,8-11,13-17,19H,6-7,12,18H2,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50370407
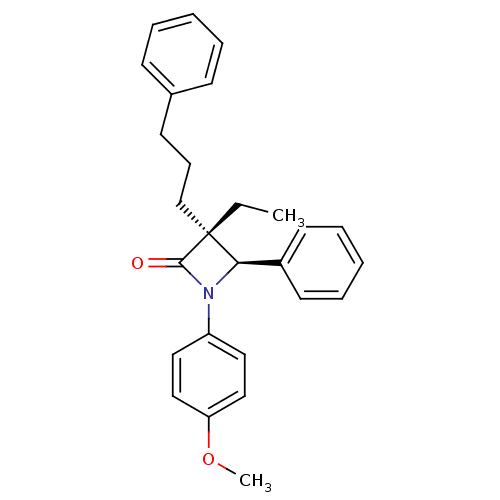
(CHEMBL49500)Show SMILES CC[C@@]1(CCCc2ccccc2)[C@H](N(C1=O)c1ccc(OC)cc1)c1ccccc1 Show InChI InChI=1S/C27H29NO2/c1-3-27(20-10-13-21-11-6-4-7-12-21)25(22-14-8-5-9-15-22)28(26(27)29)23-16-18-24(30-2)19-17-23/h4-9,11-12,14-19,25H,3,10,13,20H2,1-2H3/t25-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028867
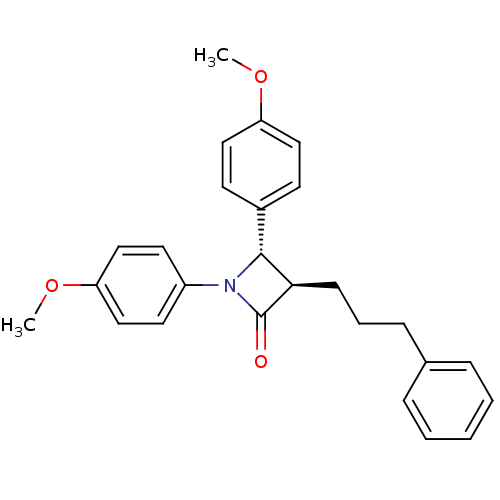
((3R,4S)-1,4-Bis-(4-methoxy-phenyl)-3-(3-phenyl-pro...)Show SMILES COc1ccc(cc1)[C@@H]1[C@@H](CCCc2ccccc2)C(=O)N1c1ccc(OC)cc1 Show InChI InChI=1S/C26H27NO3/c1-29-22-15-11-20(12-16-22)25-24(10-6-9-19-7-4-3-5-8-19)26(28)27(25)21-13-17-23(30-2)18-14-21/h3-5,7-8,11-18,24-25H,6,9-10H2,1-2H3/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50028867

((3R,4S)-1,4-Bis-(4-methoxy-phenyl)-3-(3-phenyl-pro...)Show SMILES COc1ccc(cc1)[C@@H]1[C@@H](CCCc2ccccc2)C(=O)N1c1ccc(OC)cc1 Show InChI InChI=1S/C26H27NO3/c1-29-22-15-11-20(12-16-22)25-24(10-6-9-19-7-4-3-5-8-19)26(28)27(25)21-13-17-23(30-2)18-14-21/h3-5,7-8,11-18,24-25H,6,9-10H2,1-2H3/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data