 Found 196 hits with Last Name = 'westhouse' and Initial = 'r'
Found 196 hits with Last Name = 'westhouse' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM166831
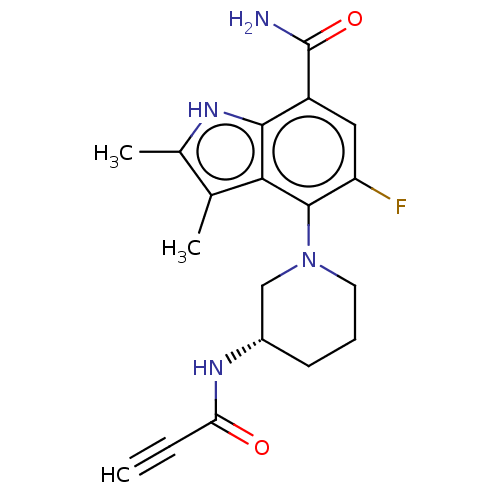
(US10604504, Example 242 | US11623921, Example 242 ...)Show SMILES Cc1[nH]c2c(cc(F)c(N3CCC[C@@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165319
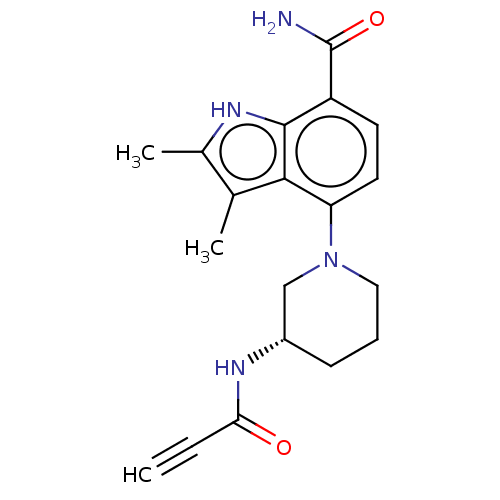
(US10604504, Example 115 | US11623921, Example 115 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM297163

(2-{8-Fluoro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(c(F)cc21)C(C)(C)O |r| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)21-14-26-27(32-17-21)22-15-24(31)23(30(2,3)37)16-25(22)36(26)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Myc proto-oncogene protein
(Homo sapiens (Human)) | BDBM445524
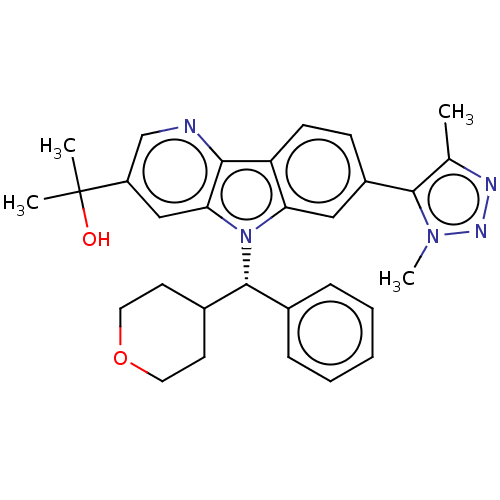
(2-{7-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...)Show SMILES Cc1nnn(C)c1-c1ccc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(cnc21)C(C)(C)O Show InChI InChI=1S/C30H33N5O2/c1-19-28(34(4)33-32-19)22-10-11-24-25(16-22)35(26-17-23(30(2,3)36)18-31-27(24)26)29(20-8-6-5-7-9-20)21-12-14-37-15-13-21/h5-11,16-18,21,29,36H,12-15H2,1-4H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MYC (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human peripheral B cells assessed as reduction in CD86 surface expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human PBMC assessed as reduction in TNFalpha expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM445527
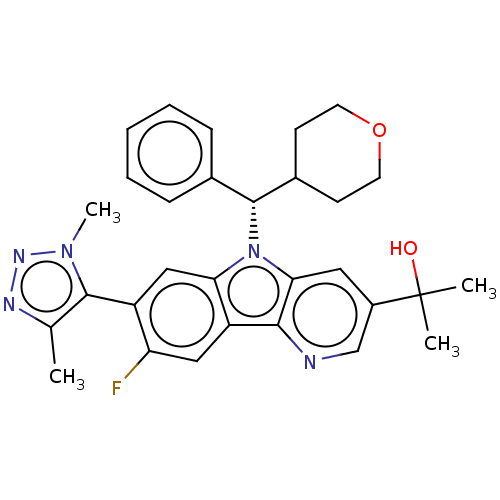
(2-[7-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-fluoro-5-[...)Show SMILES Cc1nnn(C)c1-c1cc2n([C@@H](C3CCOCC3)c3ccccc3)c3cc(cnc3c2cc1F)C(C)(C)O |r,wU:11.11,(5.87,4.1,;5.87,2.56,;7.24,1.86,;7,.34,;5.48,.1,;4.71,-1.23,;4.78,1.47,;3.3,1.87,;2.27,.73,;.76,1.05,;-.49,.14,;-.49,-1.4,;.85,-2.17,;2.18,-1.4,;3.51,-2.17,;3.51,-3.71,;2.18,-4.48,;.85,-3.71,;-1.82,-2.17,;-3.15,-1.4,;-4.49,-2.17,;-4.49,-3.71,;-3.15,-4.48,;-1.82,-3.71,;-1.73,1.05,;-3.24,.73,;-4.27,1.87,;-3.79,3.33,;-2.29,3.65,;-1.26,2.51,;.28,2.51,;1.31,3.65,;2.82,3.33,;3.85,4.48,;-5.76,1.47,;-6.85,2.56,;-6.16,-.02,;-7.24,1.07,)| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)22-16-25-23(15-24(22)31)27-26(14-21(17-32-27)30(2,3)37)36(25)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50579853
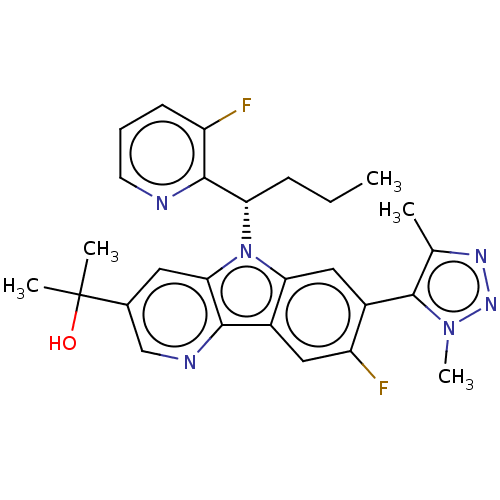
(CHEMBL5075294)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cc2n([C@@H](CCC)c3ncccc3F)c3cc(cnc3c2cc1F)C(C)(C)O |r,wD:14.18,(54.77,-34.94,;56.32,-34.94,;57.07,-36.27,;55.78,-36.06,;57.04,-33.58,;58.57,-33.38,;58.84,-31.86,;57.49,-31.13,;57.28,-29.61,;56.38,-32.2,;54.86,-31.93,;53.87,-33.1,;52.35,-32.82,;51.14,-33.77,;51.2,-35.31,;49.89,-36.12,;48.54,-35.4,;47.23,-36.21,;52.55,-36.03,;53.85,-35.22,;55.21,-35.93,;55.27,-37.48,;53.96,-38.3,;52.6,-37.57,;51.29,-38.38,;49.87,-32.9,;48.39,-33.26,;47.33,-32.16,;47.76,-30.69,;49.25,-30.33,;50.3,-31.44,;51.84,-31.37,;52.83,-30.2,;54.34,-30.47,;55.33,-29.29,;45.84,-32.53,;45.41,-34.01,;44.34,-32.93,;44.77,-31.42,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165320
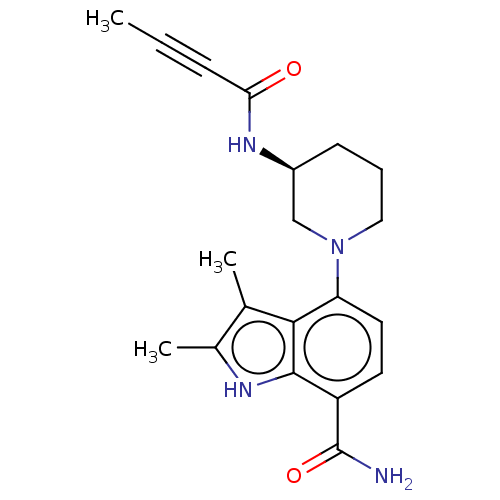
(US10604504, Example 116 | US11623921, Example 116 ...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1ccc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50579851
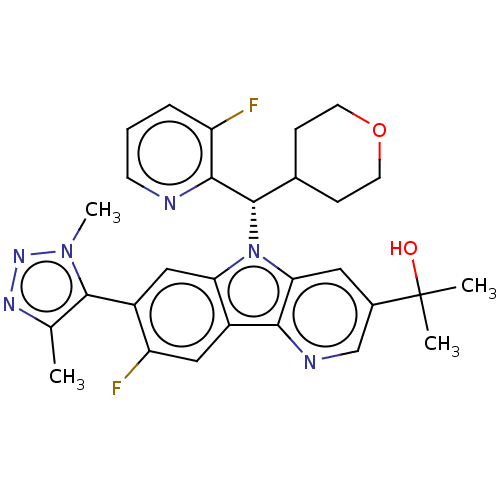
(CHEMBL5084198)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cc2n([C@@H](C3CCOCC3)c3ncccc3F)c3cc(cnc3c2cc1F)C(C)(C)O |r,wD:14.22,(13.75,-33.71,;15.3,-33.71,;16.06,-35.04,;14.76,-34.83,;16.03,-32.35,;17.55,-32.15,;17.83,-30.63,;16.47,-29.9,;16.26,-28.37,;15.36,-30.97,;13.85,-30.69,;12.85,-31.87,;11.34,-31.59,;10.13,-32.54,;10.18,-34.08,;8.87,-34.89,;7.52,-34.16,;6.22,-34.97,;6.26,-36.51,;7.62,-37.24,;8.93,-36.43,;11.54,-34.8,;12.84,-33.99,;14.19,-34.7,;14.25,-36.25,;12.94,-37.06,;11.58,-36.34,;10.27,-37.15,;8.85,-31.67,;7.37,-32.03,;6.32,-30.93,;6.75,-29.46,;8.23,-29.1,;9.29,-30.2,;10.82,-30.14,;11.81,-28.97,;13.32,-29.24,;14.31,-28.06,;4.82,-31.3,;4.39,-32.78,;3.32,-31.69,;3.75,-30.19,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50579852
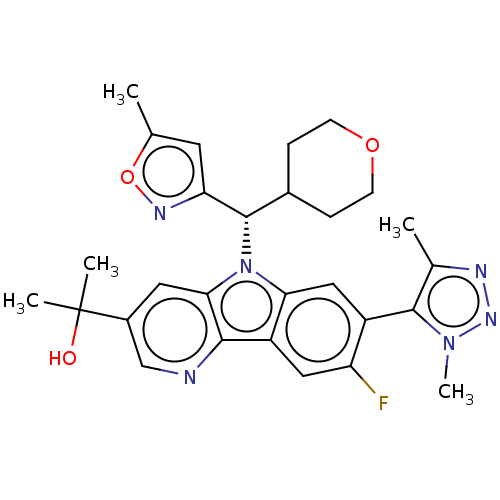
(CHEMBL5090513)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cc2n([C@@H](C3CCOCC3)c3cc(C)on3)c3cc(cnc3c2cc1F)C(C)(C)O |r,wD:14.22,(33.12,-33.94,;34.66,-33.94,;35.42,-35.27,;34.12,-35.06,;35.39,-32.58,;36.91,-32.38,;37.19,-30.86,;35.83,-30.13,;35.62,-28.61,;34.72,-31.2,;33.21,-30.93,;32.21,-32.1,;30.7,-31.82,;29.49,-32.77,;29.54,-34.31,;28.23,-35.12,;26.88,-34.39,;25.58,-35.2,;25.62,-36.74,;26.98,-37.47,;28.29,-36.66,;30.9,-35.03,;31.11,-36.55,;32.63,-36.82,;33.3,-38.2,;33.35,-35.46,;32.29,-34.35,;28.21,-31.9,;26.73,-32.26,;25.68,-31.16,;26.11,-29.69,;27.6,-29.33,;28.65,-30.44,;30.18,-30.37,;31.17,-29.2,;32.68,-29.47,;33.67,-28.29,;24.18,-31.53,;23.75,-33.01,;22.68,-31.92,;23.12,-30.42,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566615

(CHEMBL4846895)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ncccc1F)c1c(F)c(ccc21)C(C)(C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128108
BindingDB Entry DOI: 10.7270/Q26977BM |
More data for this
Ligand-Target Pair | |
Myc proto-oncogene protein
(Homo sapiens (Human)) | BDBM445528
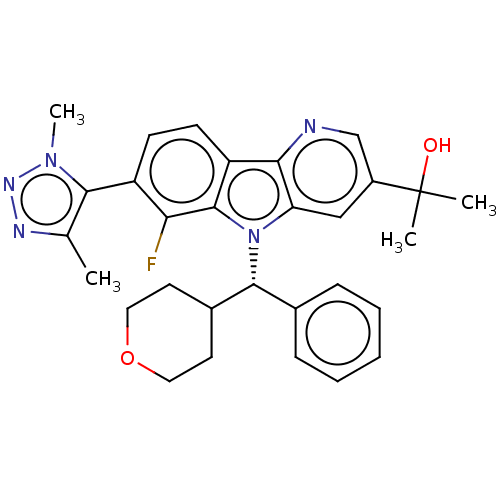
(2-[7-(Dimethyl-1H-1,2,3-triazol-5-yl)-6-fluoro-5-[...)Show SMILES Cc1nnn(C)c1-c1ccc2c3ncc(cc3n([C@@H](C3CCOCC3)c3ccccc3)c2c1F)C(C)(C)O |r,wU:18.19,(5.87,4.29,;5.87,2.75,;7.24,2.05,;7,.53,;5.48,.29,;4.71,-1.04,;4.78,1.66,;3.3,2.06,;2.82,3.52,;1.31,3.84,;.28,2.7,;-1.26,2.7,;-2.29,3.84,;-3.79,3.52,;-4.27,2.06,;-3.24,.92,;-1.73,1.24,;-.49,.33,;-.49,-1.21,;.85,-1.98,;2.18,-1.21,;3.51,-1.98,;3.51,-3.52,;2.18,-4.29,;.85,-3.52,;-1.82,-1.98,;-3.15,-1.21,;-4.49,-1.98,;-4.49,-3.52,;-3.15,-4.29,;-1.82,-3.52,;.76,1.24,;2.27,.92,;2.74,-.55,;-5.76,1.66,;-6.85,2.75,;-6.16,.17,;-7.24,1.26,)| Show InChI InChI=1S/C30H32FN5O2/c1-18-27(35(4)34-33-18)22-10-11-23-26-24(16-21(17-32-26)30(2,3)37)36(29(23)25(22)31)28(19-8-6-5-7-9-19)20-12-14-38-15-13-20/h5-11,16-17,20,28,37H,12-15H2,1-4H3/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MYC (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165463
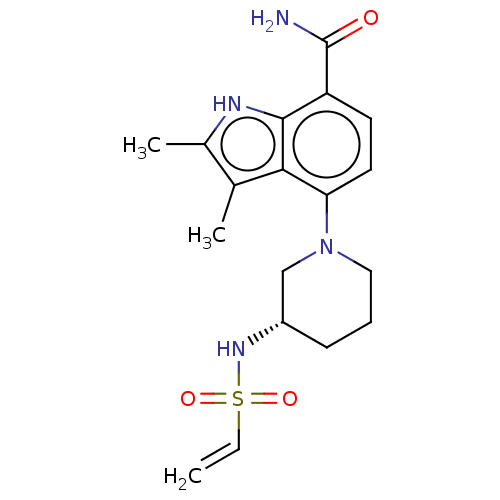
(US10604504, Example 141 | US11623921, Example 141 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@@H](C3)NS(=O)(=O)C=C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM445524

(2-{7-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...)Show SMILES Cc1nnn(C)c1-c1ccc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(cnc21)C(C)(C)O Show InChI InChI=1S/C30H33N5O2/c1-19-28(34(4)33-32-19)22-10-11-24-25(16-22)35(26-17-23(30(2,3)36)18-31-27(24)26)29(20-8-6-5-7-9-20)21-12-14-37-15-13-21/h5-11,16-18,21,29,36H,12-15H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM445515
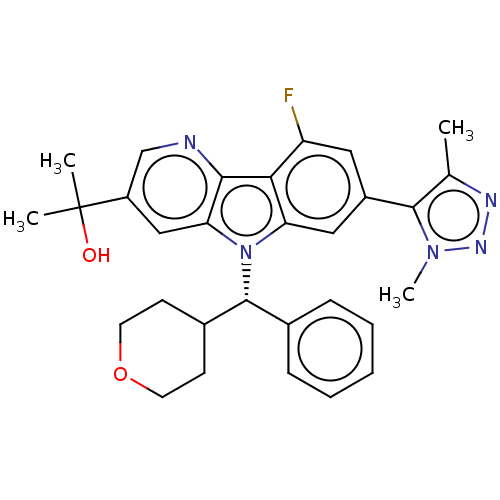
(2-[7-(Dimethyl-1H-1,2,3-triazol-5-yl)-9-fluoro-5-[...)Show SMILES Cc1nnn(C)c1-c1cc(F)c2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(cnc21)C(C)(C)O |r| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)21-14-23(31)26-24(15-21)36(25-16-22(30(2,3)37)17-32-27(25)26)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Myc proto-oncogene protein
(Homo sapiens (Human)) | BDBM50579853

(CHEMBL5075294)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cc2n([C@@H](CCC)c3ncccc3F)c3cc(cnc3c2cc1F)C(C)(C)O |r,wD:14.18,(54.77,-34.94,;56.32,-34.94,;57.07,-36.27,;55.78,-36.06,;57.04,-33.58,;58.57,-33.38,;58.84,-31.86,;57.49,-31.13,;57.28,-29.61,;56.38,-32.2,;54.86,-31.93,;53.87,-33.1,;52.35,-32.82,;51.14,-33.77,;51.2,-35.31,;49.89,-36.12,;48.54,-35.4,;47.23,-36.21,;52.55,-36.03,;53.85,-35.22,;55.21,-35.93,;55.27,-37.48,;53.96,-38.3,;52.6,-37.57,;51.29,-38.38,;49.87,-32.9,;48.39,-33.26,;47.33,-32.16,;47.76,-30.69,;49.25,-30.33,;50.3,-31.44,;51.84,-31.37,;52.83,-30.2,;54.34,-30.47,;55.33,-29.29,;45.84,-32.53,;45.41,-34.01,;44.34,-32.93,;44.77,-31.42,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MYC (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Myc proto-oncogene protein
(Homo sapiens (Human)) | BDBM297146
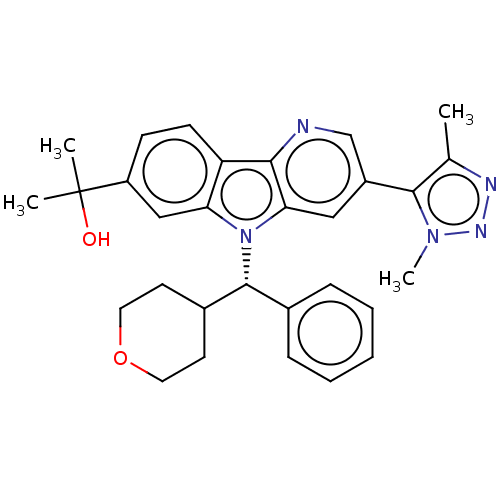
(2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(ccc21)C(C)(C)O |r| Show InChI InChI=1S/C30H33N5O2/c1-19-28(34(4)33-32-19)22-16-26-27(31-18-22)24-11-10-23(30(2,3)36)17-25(24)35(26)29(20-8-6-5-7-9-20)21-12-14-37-15-13-21/h5-11,16-18,21,29,36H,12-15H2,1-4H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MYC (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Myc proto-oncogene protein
(Homo sapiens (Human)) | BDBM445527

(2-[7-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-fluoro-5-[...)Show SMILES Cc1nnn(C)c1-c1cc2n([C@@H](C3CCOCC3)c3ccccc3)c3cc(cnc3c2cc1F)C(C)(C)O |r,wU:11.11,(5.87,4.1,;5.87,2.56,;7.24,1.86,;7,.34,;5.48,.1,;4.71,-1.23,;4.78,1.47,;3.3,1.87,;2.27,.73,;.76,1.05,;-.49,.14,;-.49,-1.4,;.85,-2.17,;2.18,-1.4,;3.51,-2.17,;3.51,-3.71,;2.18,-4.48,;.85,-3.71,;-1.82,-2.17,;-3.15,-1.4,;-4.49,-2.17,;-4.49,-3.71,;-3.15,-4.48,;-1.82,-3.71,;-1.73,1.05,;-3.24,.73,;-4.27,1.87,;-3.79,3.33,;-2.29,3.65,;-1.26,2.51,;.28,2.51,;1.31,3.65,;2.82,3.33,;3.85,4.48,;-5.76,1.47,;-6.85,2.56,;-6.16,-.02,;-7.24,1.07,)| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)22-16-25-23(15-24(22)31)27-26(14-21(17-32-27)30(2,3)37)36(25)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MYC (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297146

(2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(ccc21)C(C)(C)O |r| Show InChI InChI=1S/C30H33N5O2/c1-19-28(34(4)33-32-19)22-16-26-27(31-18-22)24-11-10-23(30(2,3)36)17-25(24)35(26)29(20-8-6-5-7-9-20)21-12-14-37-15-13-21/h5-11,16-18,21,29,36H,12-15H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297236

(2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-methoxy-5-...)Show SMILES COc1cc2c(cc1C(C)(C)O)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(cnc21)-c1c(C)nnn1C |r| Show InChI InChI=1S/C31H35N5O3/c1-19-29(35(4)34-33-19)22-15-26-28(32-18-22)23-16-27(38-5)24(31(2,3)37)17-25(23)36(26)30(20-9-7-6-8-10-20)21-11-13-39-14-12-21/h6-10,15-18,21,30,37H,11-14H2,1-5H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165318

(US10604504, Example 114 | US11623921, Example 114 ...)Show SMILES Cc1[nH]c2c(ccc(NC3CCN(CC3)C(=O)C#C)c2c1C)C(N)=O Show InChI InChI=1S/C19H22N4O2/c1-4-16(24)23-9-7-13(8-10-23)22-15-6-5-14(19(20)25)18-17(15)11(2)12(3)21-18/h1,5-6,13,21-22H,7-10H2,2-3H3,(H2,20,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297163

(2-{8-Fluoro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(c(F)cc21)C(C)(C)O |r| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)21-14-26-27(32-17-21)22-15-24(31)23(30(2,3)37)16-25(22)36(26)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297070
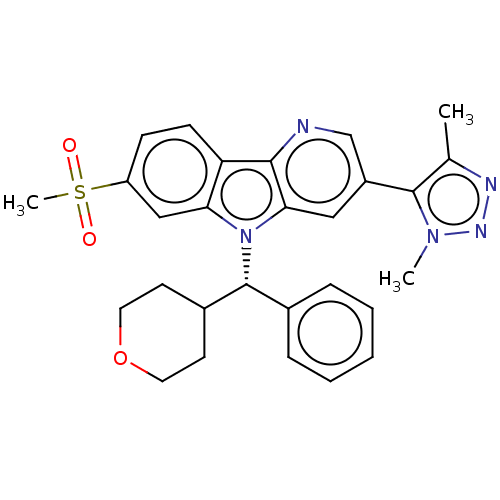
(5-{7-Methanesulfonyl-5-[(S)-oxan-4-yl(phenyl)methy...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(ccc21)S(C)(=O)=O |r| Show InChI InChI=1S/C28H29N5O3S/c1-18-27(32(2)31-30-18)21-15-25-26(29-17-21)23-10-9-22(37(3,34)35)16-24(23)33(25)28(19-7-5-4-6-8-19)20-11-13-36-14-12-20/h4-10,15-17,20,28H,11-14H2,1-3H3/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566615

(CHEMBL4846895)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ncccc1F)c1c(F)c(ccc21)C(C)(C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) assessed as reduction in c-Myc expression |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128108
BindingDB Entry DOI: 10.7270/Q26977BM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human memory B cells assessed as reduction in CD86 surface expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566614
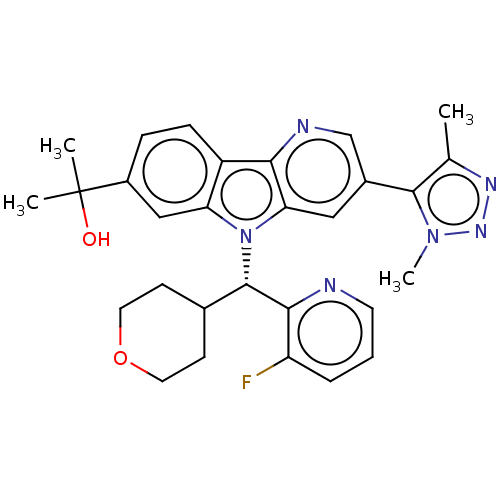
(CHEMBL4848871)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ncccc1F)c1cc(ccc21)C(C)(C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128108
BindingDB Entry DOI: 10.7270/Q26977BM |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297163

(2-{8-Fluoro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(c(F)cc21)C(C)(C)O |r| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)21-14-26-27(32-17-21)22-15-24(31)23(30(2,3)37)16-25(22)36(26)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128108
BindingDB Entry DOI: 10.7270/Q26977BM |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 1
(Homo sapiens (Human)) | BDBM50074796

(CHEMBL3410169)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2ccc(F)cc2F)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C29H25F5N4O3/c1-38-22-10-6-5-9-19(22)24(16-7-3-2-4-8-16)36-26(28(38)41)37-27(40)20(13-14-29(32,33)34)23(25(35)39)18-12-11-17(30)15-21(18)31/h2-12,15,20,23,26H,13-14H2,1H3,(H2,35,39)(H,37,40)/t20-,23+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297144

(2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-9-methoxy-5-...)Show SMILES COc1cc(cc2n([C@@H](C3CCOCC3)c3ccccc3)c3cc(cnc3c12)-c1c(C)nnn1C)C(C)(C)O |r| Show InChI InChI=1S/C31H35N5O3/c1-19-29(35(4)34-33-19)22-15-25-28(32-18-22)27-24(16-23(31(2,3)37)17-26(27)38-5)36(25)30(20-9-7-6-8-10-20)21-11-13-39-14-12-21/h6-10,15-18,21,30,37H,11-14H2,1-5H3/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Myc proto-oncogene protein
(Homo sapiens (Human)) | BDBM445527

(2-[7-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-fluoro-5-[...)Show SMILES Cc1nnn(C)c1-c1cc2n([C@@H](C3CCOCC3)c3ccccc3)c3cc(cnc3c2cc1F)C(C)(C)O |r,wU:11.11,(5.87,4.1,;5.87,2.56,;7.24,1.86,;7,.34,;5.48,.1,;4.71,-1.23,;4.78,1.47,;3.3,1.87,;2.27,.73,;.76,1.05,;-.49,.14,;-.49,-1.4,;.85,-2.17,;2.18,-1.4,;3.51,-2.17,;3.51,-3.71,;2.18,-4.48,;.85,-3.71,;-1.82,-2.17,;-3.15,-1.4,;-4.49,-2.17,;-4.49,-3.71,;-3.15,-4.48,;-1.82,-3.71,;-1.73,1.05,;-3.24,.73,;-4.27,1.87,;-3.79,3.33,;-2.29,3.65,;-1.26,2.51,;.28,2.51,;1.31,3.65,;2.82,3.33,;3.85,4.48,;-5.76,1.47,;-6.85,2.56,;-6.16,-.02,;-7.24,1.07,)| Show InChI InChI=1S/C30H32FN5O2/c1-18-28(35(4)34-33-18)22-16-25-23(15-24(22)31)27-26(14-21(17-32-27)30(2,3)37)36(25)29(19-8-6-5-7-9-19)20-10-12-38-13-11-20/h5-9,14-17,20,29,37H,10-13H2,1-4H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-MYC (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128376
BindingDB Entry DOI: 10.7270/Q2KS6WD7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566614

(CHEMBL4848871)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ncccc1F)c1cc(ccc21)C(C)(C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) assessed as reduction in c-Myc expression |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128108
BindingDB Entry DOI: 10.7270/Q26977BM |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50566613

(CHEMBL4864173)Show SMILES [2H]C([2H])([2H])c1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccc(Cl)cn1)c1cc(ccc21)C(C)(C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128108
BindingDB Entry DOI: 10.7270/Q26977BM |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 3
(Homo sapiens (Human)) | BDBM50074771

(CHEMBL3410153)Show SMILES CCC(CC)[C@@H]([C@@H](CCC(F)(F)F)C(=O)N[C@H]1N=C(c2ccccc2)c2ccccc2N(C)C1=O)C(N)=O |r,t:17| Show InChI InChI=1S/C28H33F3N4O3/c1-4-17(5-2)22(24(32)36)20(15-16-28(29,30)31)26(37)34-25-27(38)35(3)21-14-10-9-13-19(21)23(33-25)18-11-7-6-8-12-18/h6-14,17,20,22,25H,4-5,15-16H2,1-3H3,(H2,32,36)(H,34,37)/t20-,22+,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297162
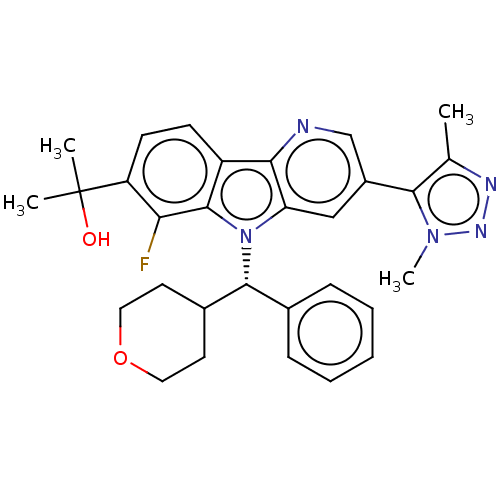
(2-{6-Fluoro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1c(F)c(ccc21)C(C)(C)O |r| Show InChI InChI=1S/C30H32FN5O2/c1-18-27(35(4)34-33-18)21-16-24-26(32-17-21)22-10-11-23(30(2,3)37)25(31)29(22)36(24)28(19-8-6-5-7-9-19)20-12-14-38-15-13-20/h5-11,16-17,20,28,37H,12-15H2,1-4H3/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 1
(Homo sapiens (Human)) | BDBM50074818

(CHEMBL3410163)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2cccc(c2)C(F)(F)F)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C30H26F6N4O3/c1-40-22-13-6-5-12-20(22)24(17-8-3-2-4-9-17)38-26(28(40)43)39-27(42)21(14-15-29(31,32)33)23(25(37)41)18-10-7-11-19(16-18)30(34,35)36/h2-13,16,21,23,26H,14-15H2,1H3,(H2,37,41)(H,39,42)/t21-,23+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 1
(Homo sapiens (Human)) | BDBM50074771

(CHEMBL3410153)Show SMILES CCC(CC)[C@@H]([C@@H](CCC(F)(F)F)C(=O)N[C@H]1N=C(c2ccccc2)c2ccccc2N(C)C1=O)C(N)=O |r,t:17| Show InChI InChI=1S/C28H33F3N4O3/c1-4-17(5-2)22(24(32)36)20(15-16-28(29,30)31)26(37)34-25-27(38)35(3)21-14-10-9-13-19(21)23(33-25)18-11-7-6-8-12-18/h6-14,17,20,22,25H,4-5,15-16H2,1-3H3,(H2,32,36)(H,34,37)/t20-,22+,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 3
(Homo sapiens (Human)) | BDBM50074799

(CHEMBL3410167)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2ccc(F)cc2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C29H26F4N4O3/c1-37-22-10-6-5-9-20(22)24(18-7-3-2-4-8-18)35-26(28(37)40)36-27(39)21(15-16-29(31,32)33)23(25(34)38)17-11-13-19(30)14-12-17/h2-14,21,23,26H,15-16H2,1H3,(H2,34,38)(H,36,39)/t21-,23+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 3
(Homo sapiens (Human)) | BDBM50074796

(CHEMBL3410169)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2ccc(F)cc2F)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C29H25F5N4O3/c1-38-22-10-6-5-9-19(22)24(16-7-3-2-4-8-16)36-26(28(38)41)37-27(40)20(13-14-29(32,33)34)23(25(35)39)18-12-11-17(30)15-21(18)31/h2-12,15,20,23,26H,13-14H2,1H3,(H2,35,39)(H,37,40)/t20-,23+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 2
(Homo sapiens (Human)) | BDBM50074694
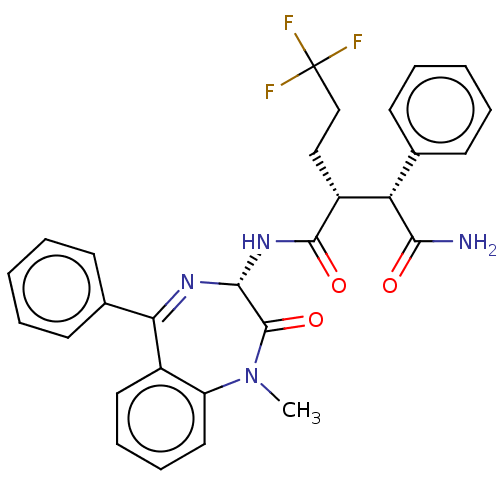
(CHEMBL3410161)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2ccccc2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C29H27F3N4O3/c1-36-22-15-9-8-14-20(22)24(19-12-6-3-7-13-19)34-26(28(36)39)35-27(38)21(16-17-29(30,31)32)23(25(33)37)18-10-4-2-5-11-18/h2-15,21,23,26H,16-17H2,1H3,(H2,33,37)(H,35,38)/t21-,23+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch2 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50586606
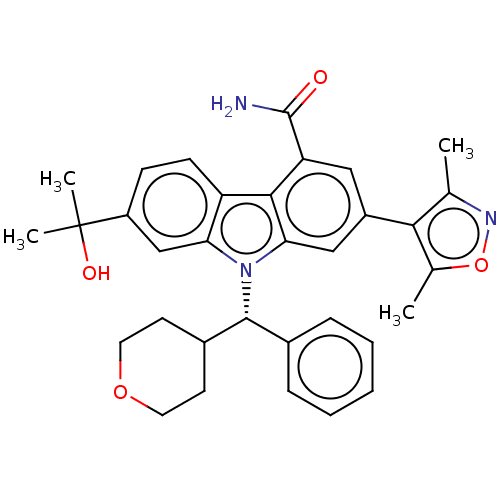
(CHEMBL5080354)Show SMILES Cc1noc(C)c1-c1cc(C(N)=O)c2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(ccc21)C(C)(C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRD4 (1 to 477 residues) measured after 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00625
BindingDB Entry DOI: 10.7270/Q2HT2T7C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neurogenic locus notch homolog protein 3
(Homo sapiens (Human)) | BDBM50074693

(CHEMBL3410162)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C30H29F3N4O3/c1-18-9-8-12-20(17-18)24(26(34)38)22(15-16-30(31,32)33)28(39)36-27-29(40)37(2)23-14-7-6-13-21(23)25(35-27)19-10-4-3-5-11-19/h3-14,17,22,24,27H,15-16H2,1-2H3,(H2,34,38)(H,36,39)/t22-,24+,27-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297146

(2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(ccc21)C(C)(C)O |r| Show InChI InChI=1S/C30H33N5O2/c1-19-28(34(4)33-32-19)22-16-26-27(31-18-22)24-11-10-23(30(2,3)36)17-25(24)35(26)29(20-8-6-5-7-9-20)21-12-14-37-15-13-21/h5-11,16-18,21,29,36H,12-15H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM297268
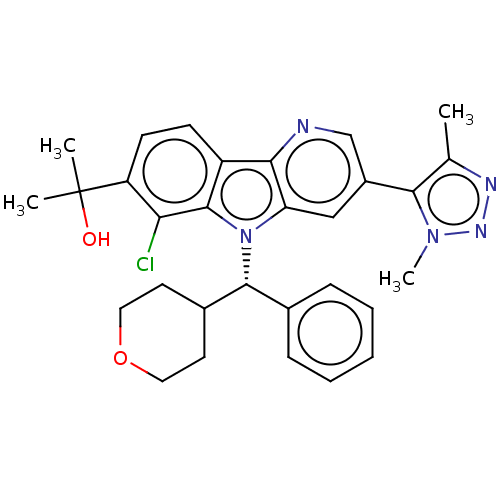
(2-{6-Chloro-3-[4-(2H3)methyl-1-methyl-1H-1,2,3-tri...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1c(Cl)c(ccc21)C(C)(C)O |r| Show InChI InChI=1S/C30H32ClN5O2/c1-18-27(35(4)34-33-18)21-16-24-26(32-17-21)22-10-11-23(30(2,3)37)25(31)29(22)36(24)28(19-8-6-5-7-9-19)20-12-14-38-15-13-20/h5-11,16-17,20,28,37H,12-15H2,1-4H3/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00219
BindingDB Entry DOI: 10.7270/Q20R9TDX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM297146

(2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...)Show SMILES Cc1nnn(C)c1-c1cnc2c(c1)n([C@@H](C1CCOCC1)c1ccccc1)c1cc(ccc21)C(C)(C)O |r| Show InChI InChI=1S/C30H33N5O2/c1-19-28(34(4)33-32-19)22-16-26-27(31-18-22)24-11-10-23(30(2,3)36)17-25(24)35(26)29(20-8-6-5-7-9-20)21-12-14-37-15-13-21/h5-11,16-18,21,29,36H,12-15H2,1-4H3/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRD3 measured by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00625
BindingDB Entry DOI: 10.7270/Q2HT2T7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data