 Found 33 hits with Last Name = 'wickson' and Initial = 'kf'
Found 33 hits with Last Name = 'wickson' and Initial = 'kf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35948
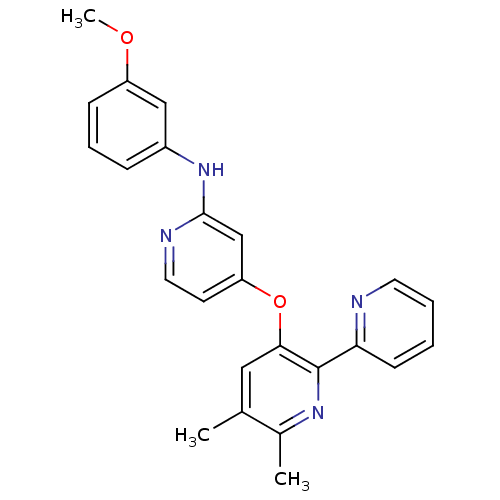
(4-pyridinoxy-2-anilinopyridine-based compound, 12)Show SMILES COc1cccc(Nc2cc(Oc3cc(C)c(C)nc3-c3ccccn3)ccn2)c1 Show InChI InChI=1S/C24H22N4O2/c1-16-13-22(24(27-17(16)2)21-9-4-5-11-25-21)30-20-10-12-26-23(15-20)28-18-7-6-8-19(14-18)29-3/h4-15H,1-3H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | 32 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35949
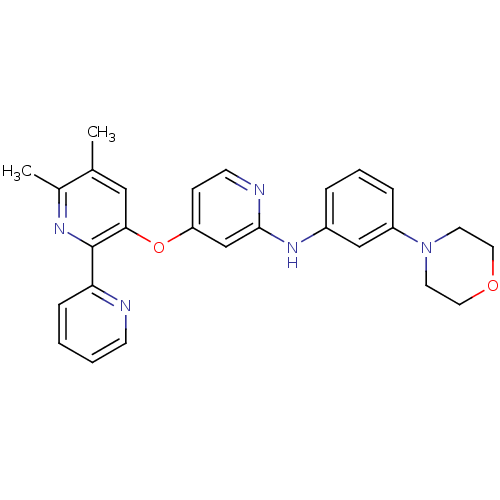
(4-pyridinoxy-2-anilinopyridine-based compound, 13)Show SMILES Cc1cc(Oc2ccnc(Nc3cccc(c3)N3CCOCC3)c2)c(nc1C)-c1ccccn1 Show InChI InChI=1S/C27H27N5O2/c1-19-16-25(27(30-20(19)2)24-8-3-4-10-28-24)34-23-9-11-29-26(18-23)31-21-6-5-7-22(17-21)32-12-14-33-15-13-32/h3-11,16-18H,12-15H2,1-2H3,(H,29,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | 62 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35946
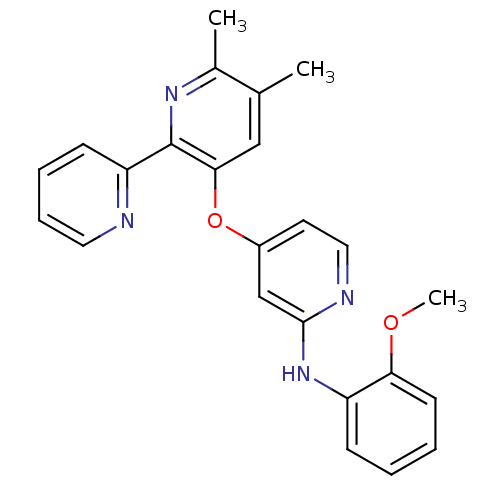
(4-pyridinoxy-2-anilinopyridine-based compound, 10)Show SMILES COc1ccccc1Nc1cc(Oc2cc(C)c(C)nc2-c2ccccn2)ccn1 Show InChI InChI=1S/C24H22N4O2/c1-16-14-22(24(27-17(16)2)20-9-6-7-12-25-20)30-18-11-13-26-23(15-18)28-19-8-4-5-10-21(19)29-3/h4-15H,1-3H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | 114 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35953

(4-pyridinoxy-2-anilinopyridine-based compound, 17)Show SMILES CN(C)C(=O)c1ccc(Nc2cc(Oc3cc(C)c(C)nc3-c3ccccn3)ccn2)cc1 Show InChI InChI=1S/C26H25N5O2/c1-17-15-23(25(29-18(17)2)22-7-5-6-13-27-22)33-21-12-14-28-24(16-21)30-20-10-8-19(9-11-20)26(32)31(3)4/h5-16H,1-4H3,(H,28,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | 31 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35941

(4-pyridinoxy-2-anilinopyridine-based compound, 5)Show SMILES COc1cc(Nc2cc(Oc3ccc(C)nc3-c3ccccn3)ccn2)cc(OC)c1OC Show InChI InChI=1S/C25H24N4O4/c1-16-8-9-20(24(28-16)19-7-5-6-11-26-19)33-18-10-12-27-23(15-18)29-17-13-21(30-2)25(32-4)22(14-17)31-3/h5-15H,1-4H3,(H,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | 20 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35952
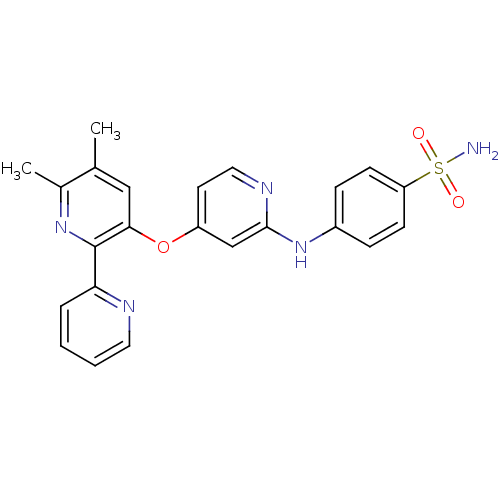
(4-pyridinoxy-2-anilinopyridine-based compound, 16)Show SMILES Cc1cc(Oc2ccnc(Nc3ccc(cc3)S(N)(=O)=O)c2)c(nc1C)-c1ccccn1 Show InChI InChI=1S/C23H21N5O3S/c1-15-13-21(23(27-16(15)2)20-5-3-4-11-25-20)31-18-10-12-26-22(14-18)28-17-6-8-19(9-7-17)32(24,29)30/h3-14H,1-2H3,(H,26,28)(H2,24,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | 27 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35950
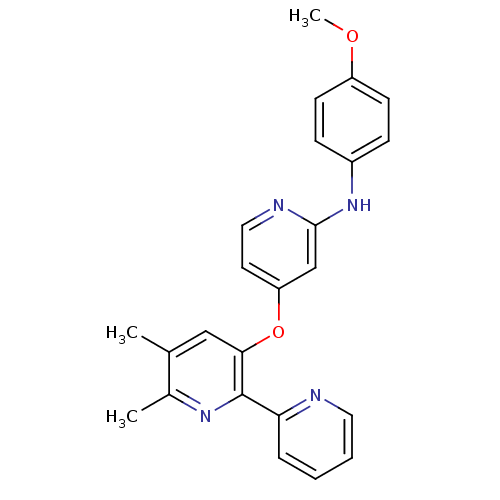
(4-pyridinoxy-2-anilinopyridine-based compound, 14)Show SMILES COc1ccc(Nc2cc(Oc3cc(C)c(C)nc3-c3ccccn3)ccn2)cc1 Show InChI InChI=1S/C24H22N4O2/c1-16-14-22(24(27-17(16)2)21-6-4-5-12-25-21)30-20-11-13-26-23(15-20)28-18-7-9-19(29-3)10-8-18/h4-15H,1-3H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | 35 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35942
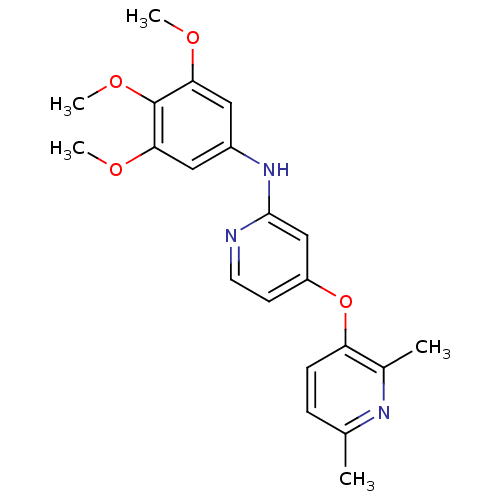
(4-pyridinoxy-2-anilinopyridine-based compound, 6)Show InChI InChI=1S/C21H23N3O4/c1-13-6-7-17(14(2)23-13)28-16-8-9-22-20(12-16)24-15-10-18(25-3)21(27-5)19(11-15)26-4/h6-12H,1-5H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | 21 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35939
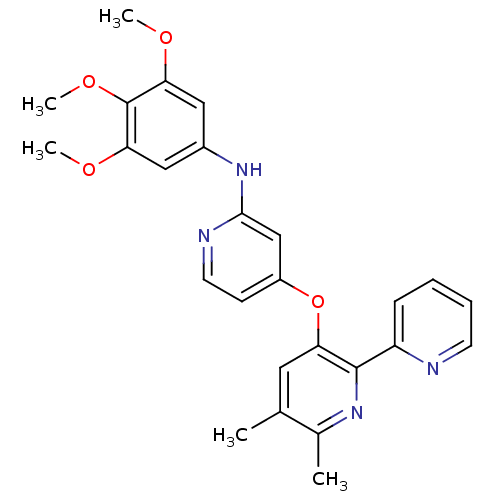
(4-pyridinoxy-2-anilinopyridine-based compound, 1)Show SMILES COc1cc(Nc2cc(Oc3cc(C)c(C)nc3-c3ccccn3)ccn2)cc(OC)c1OC Show InChI InChI=1S/C26H26N4O4/c1-16-12-21(25(29-17(16)2)20-8-6-7-10-27-20)34-19-9-11-28-24(15-19)30-18-13-22(31-3)26(33-5)23(14-18)32-4/h6-15H,1-5H3,(H,28,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 44 | n/a | 55 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35955
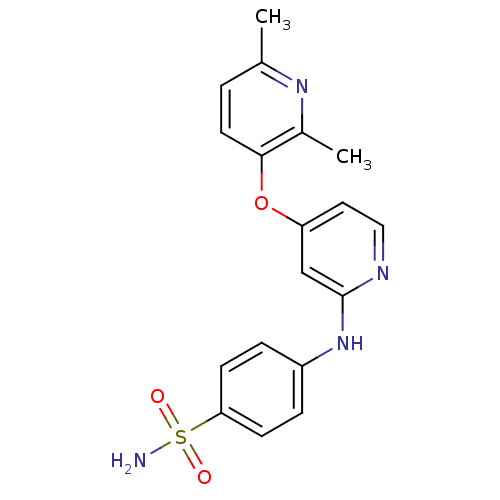
(4-pyridinoxy-2-anilinopyridine-based compound, 19)Show SMILES Cc1ccc(Oc2ccnc(Nc3ccc(cc3)S(N)(=O)=O)c2)c(C)n1 Show InChI InChI=1S/C18H18N4O3S/c1-12-3-8-17(13(2)21-12)25-15-9-10-20-18(11-15)22-14-4-6-16(7-5-14)26(19,23)24/h3-11H,1-2H3,(H,20,22)(H2,19,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | 22 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35945
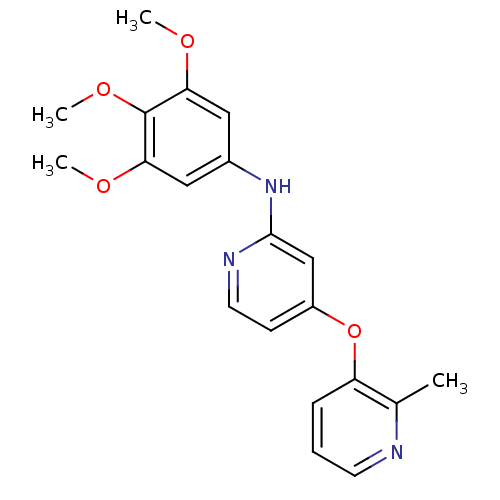
(4-pyridinoxy-2-anilinopyridine-based compound, 9)Show InChI InChI=1S/C20H21N3O4/c1-13-16(6-5-8-21-13)27-15-7-9-22-19(12-15)23-14-10-17(24-2)20(26-4)18(11-14)25-3/h5-12H,1-4H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | 628 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35944
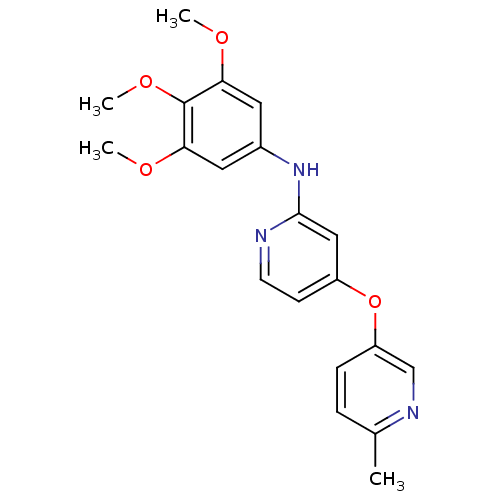
(4-pyridinoxy-2-anilinopyridine-based compound, 8)Show InChI InChI=1S/C20H21N3O4/c1-13-5-6-16(12-22-13)27-15-7-8-21-19(11-15)23-14-9-17(24-2)20(26-4)18(10-14)25-3/h5-12H,1-4H3,(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | 282 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35951
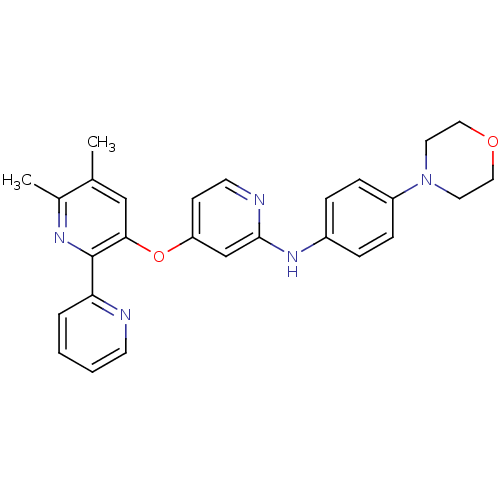
(4-pyridinoxy-2-anilinopyridine-based compound, 15)Show SMILES Cc1cc(Oc2ccnc(Nc3ccc(cc3)N3CCOCC3)c2)c(nc1C)-c1ccccn1 Show InChI InChI=1S/C27H27N5O2/c1-19-17-25(27(30-20(19)2)24-5-3-4-11-28-24)34-23-10-12-29-26(18-23)31-21-6-8-22(9-7-21)32-13-15-33-16-14-32/h3-12,17-18H,13-16H2,1-2H3,(H,29,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | 146 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35954
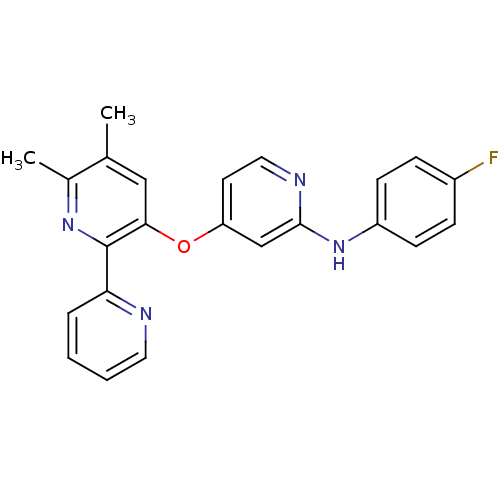
(4-pyridinoxy-2-anilinopyridine-based compound, 18)Show SMILES Cc1cc(Oc2ccnc(Nc3ccc(F)cc3)c2)c(nc1C)-c1ccccn1 Show InChI InChI=1S/C23H19FN4O/c1-15-13-21(23(27-16(15)2)20-5-3-4-11-25-20)29-19-10-12-26-22(14-19)28-18-8-6-17(24)7-9-18/h3-14H,1-2H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | 63 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35943

(4-pyridinoxy-2-anilinopyridine-based compound, 7)Show InChI InChI=1S/C19H19N3O4/c1-23-16-9-13(10-17(24-2)19(16)25-3)22-18-11-14(6-8-21-18)26-15-5-4-7-20-12-15/h4-12H,1-3H3,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | >5.80E+3 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM35947
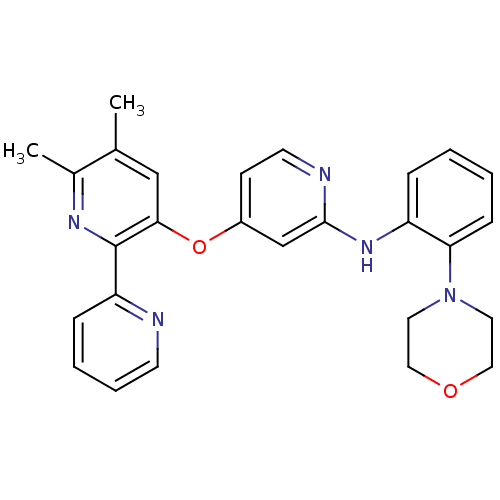
(4-pyridinoxy-2-anilinopyridine-based compound, 11)Show SMILES Cc1cc(Oc2ccnc(Nc3ccccc3N3CCOCC3)c2)c(nc1C)-c1ccccn1 Show InChI InChI=1S/C27H27N5O2/c1-19-17-25(27(30-20(19)2)23-8-5-6-11-28-23)34-21-10-12-29-26(18-21)31-22-7-3-4-9-24(22)32-13-15-33-16-14-32/h3-12,17-18H,13-16H2,1-2H3,(H,29,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 739 | n/a | >3.00E+3 | n/a | n/a | 7.4 | 23 |
AstraZeneca
| Assay Description
A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... |
J Med Chem 52: 7901-5 (2009)
Article DOI: 10.1021/jm900807w
BindingDB Entry DOI: 10.7270/Q2KS6PXG |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615236
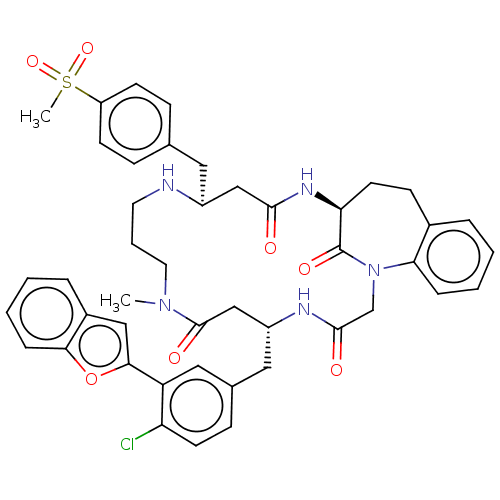
(CHEMBL5267198)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(c3)-c3cc4ccccc4o3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615235
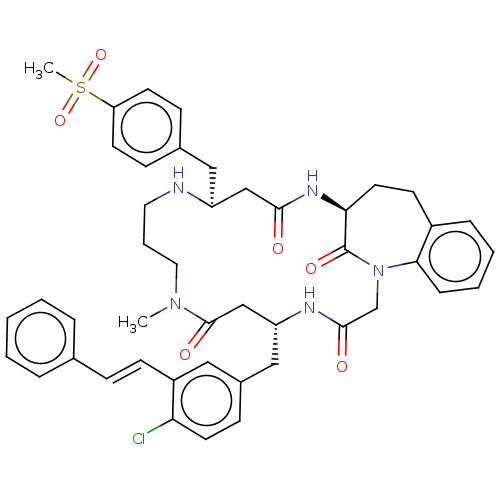
(CHEMBL5269798)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(\C=C\c4ccccc4)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615234
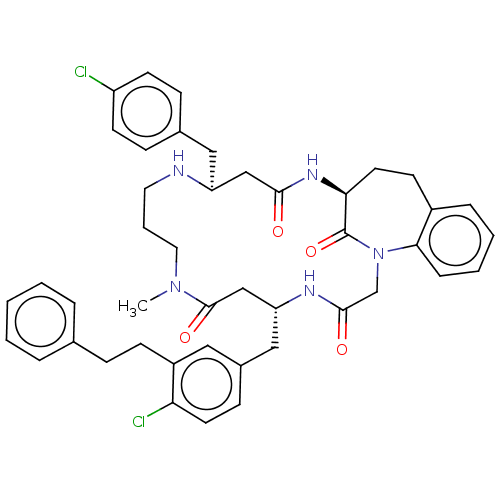
(CHEMBL5282328)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(CCc4ccccc4)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615233

(CHEMBL5280275)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(c3)-c3cc4ccccc4o3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615232

(CHEMBL5280696)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(\C=C\c4ccccc4)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508948
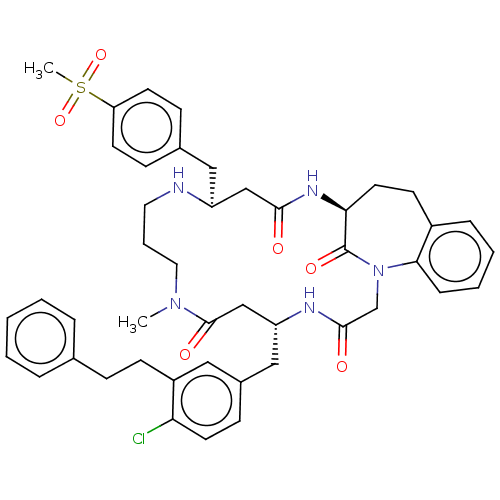
(CHEMBL4473598)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(CCc4ccccc4)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C45H52ClN5O6S/c1-50-24-8-23-47-36(26-32-14-19-38(20-15-32)58(2,56)57)28-42(52)49-40-22-18-34-11-6-7-12-41(34)51(45(40)55)30-43(53)48-37(29-44(50)54)27-33-16-21-39(46)35(25-33)17-13-31-9-4-3-5-10-31/h3-7,9-12,14-16,19-21,25,36-37,40,47H,8,13,17-18,22-24,26-30H2,1-2H3,(H,48,53)(H,49,52)/t36-,37+,40-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508950

(CHEMBL4472439)Show SMILES C[C@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508940
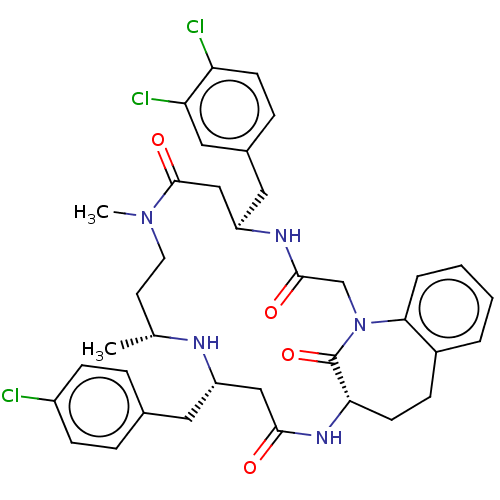
(CHEMBL4582512)Show SMILES C[C@@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28+,29-,32+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615231

(CHEMBL5266160)Show SMILES CN1CCCCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615230

(CHEMBL5268588)Show SMILES CN1CCCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508947
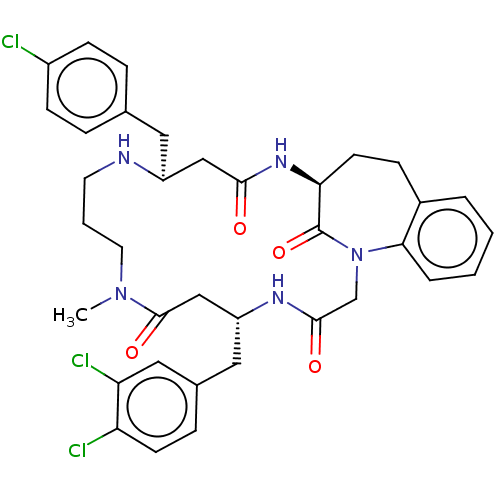
(CHEMBL4460550)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C36H40Cl3N5O4/c1-43-16-4-15-40-27(17-23-7-11-26(37)12-8-23)20-33(45)42-31-14-10-25-5-2-3-6-32(25)44(36(31)48)22-34(46)41-28(21-35(43)47)18-24-9-13-29(38)30(39)19-24/h2-3,5-9,11-13,19,27-28,31,40H,4,10,14-18,20-22H2,1H3,(H,41,46)(H,42,45)/t27-,28+,31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508936
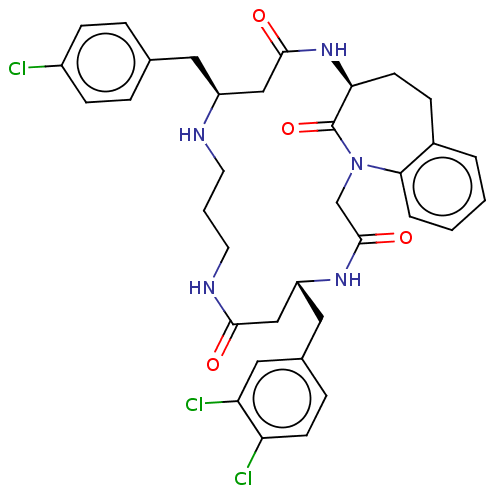
(CHEMBL4590345)Show SMILES Clc1ccc(C[C@H]2CC(=O)N[C@H]3CCc4ccccc4N(CC(=O)N[C@H](Cc4ccc(Cl)c(Cl)c4)CC(=O)NCCCN2)C3=O)cc1 |r| Show InChI InChI=1S/C35H38Cl3N5O4/c36-25-10-6-22(7-11-25)16-26-19-33(45)42-30-13-9-24-4-1-2-5-31(24)43(35(30)47)21-34(46)41-27(20-32(44)40-15-3-14-39-26)17-23-8-12-28(37)29(38)18-23/h1-2,4-8,10-12,18,26-27,30,39H,3,9,13-17,19-21H2,(H,40,44)(H,41,46)(H,42,45)/t26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615229
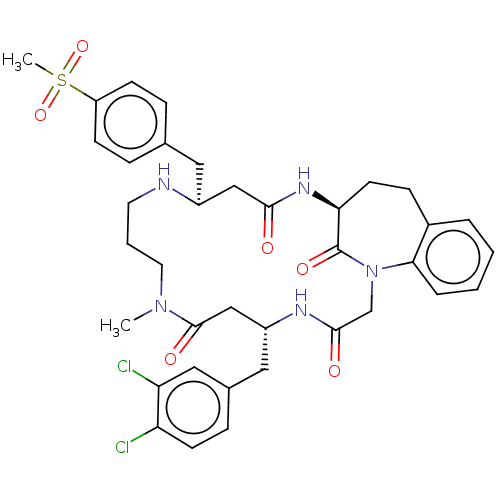
(CHEMBL5282122)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615228
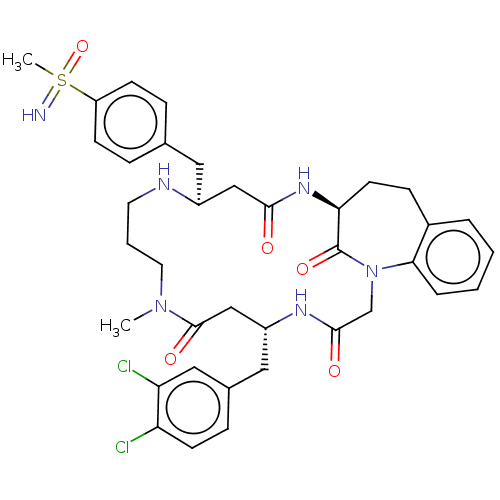
(CHEMBL5277654)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=N)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615227
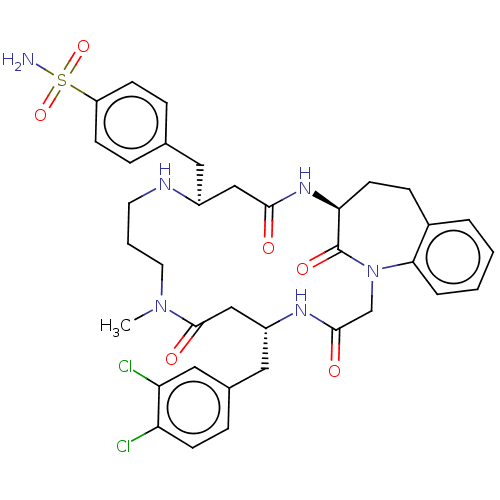
(CHEMBL5288551)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(N)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615237
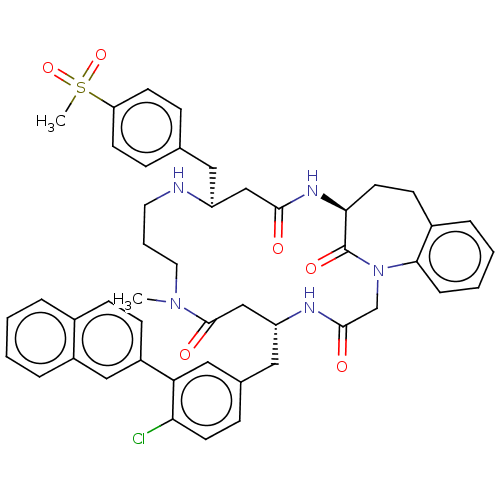
(CHEMBL5275336)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(c3)-c3ccc4ccccc4c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615226

(CHEMBL5269652)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)[S+](C)[O-])CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data