 Found 1248 hits with Last Name = 'wolff' and Initial = 'm'
Found 1248 hits with Last Name = 'wolff' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313984
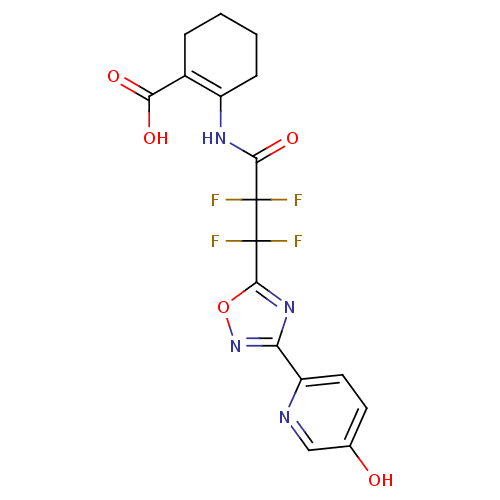
(2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C(F)(F)C(F)(F)c1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H14F4N4O5/c18-16(19,14(29)23-10-4-2-1-3-9(10)13(27)28)17(20,21)15-24-12(25-30-15)11-6-5-8(26)7-22-11/h5-7,26H,1-4H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977
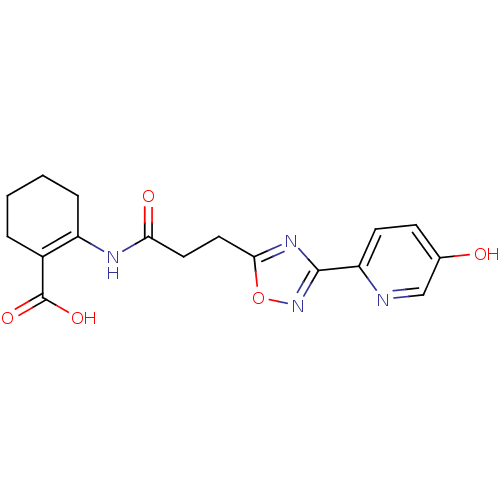
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313976
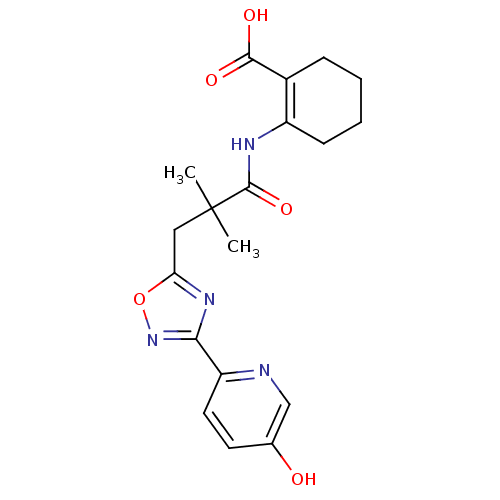
(2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES CC(C)(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:21| Show InChI InChI=1S/C19H22N4O5/c1-19(2,18(27)21-13-6-4-3-5-12(13)17(25)26)9-15-22-16(23-28-15)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533
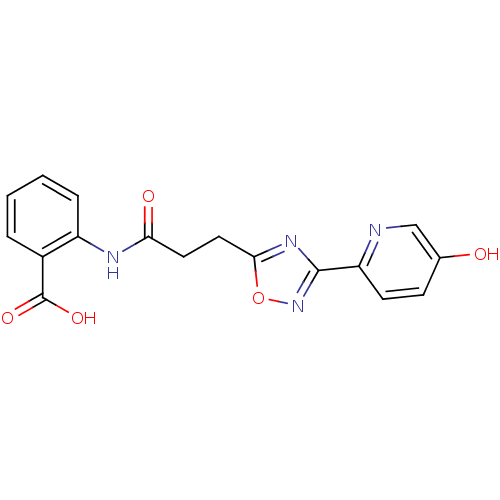
(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313979
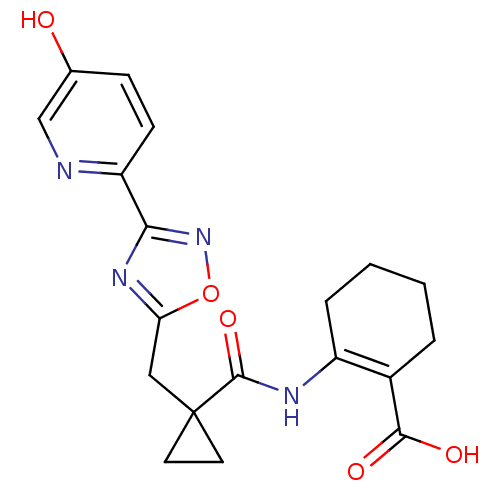
(2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C1(Cc2nc(no2)-c2ccc(O)cn2)CC1 |t:3| Show InChI InChI=1S/C19H20N4O5/c24-11-5-6-14(20-10-11)16-22-15(28-23-16)9-19(7-8-19)18(27)21-13-4-2-1-3-12(13)17(25)26/h5-6,10,24H,1-4,7-9H2,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313981
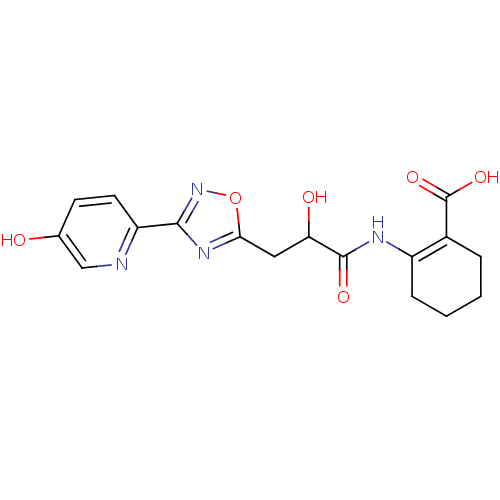
(CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...)Show SMILES OC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C17H18N4O6/c22-9-5-6-12(18-8-9)15-20-14(27-21-15)7-13(23)16(24)19-11-4-2-1-3-10(11)17(25)26/h5-6,8,13,22-23H,1-4,7H2,(H,19,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313982
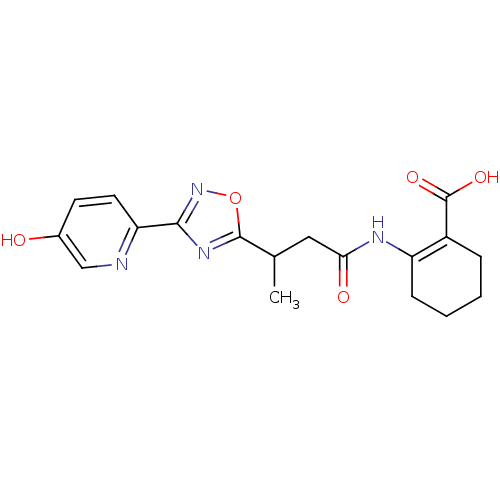
(CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...)Show SMILES CC(CC(=O)NC1=C(CCCC1)C(O)=O)c1nc(no1)-c1ccc(O)cn1 |t:6| Show InChI InChI=1S/C18H20N4O5/c1-10(8-15(24)20-13-5-3-2-4-12(13)18(25)26)17-21-16(22-27-17)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533

(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313983
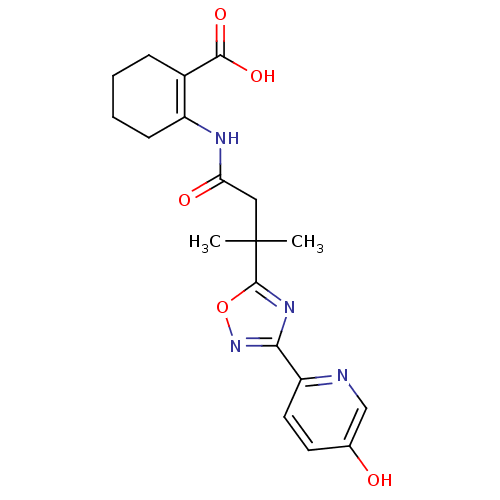
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(C)(CC(=O)NC1=C(CCCC1)C(O)=O)c1nc(no1)-c1ccc(O)cn1 |t:7| Show InChI InChI=1S/C19H22N4O5/c1-19(2,9-15(25)21-13-6-4-3-5-12(13)17(26)27)18-22-16(23-28-18)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313980
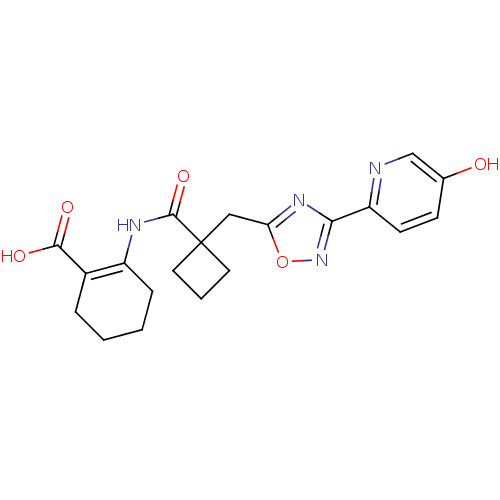
(2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C1(Cc2nc(no2)-c2ccc(O)cn2)CCC1 |t:3| Show InChI InChI=1S/C20H22N4O5/c25-12-6-7-15(21-11-12)17-23-16(29-24-17)10-20(8-3-9-20)19(28)22-14-5-2-1-4-13(14)18(26)27/h6-7,11,25H,1-5,8-10H2,(H,22,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523582

(CHEMBL4447657)Show SMILES CN1CCN(CC1)c1ccc(cn1)-c1ccc(cc1)-c1nc2cc(ccc2[nH]1)C(F)(F)F Show InChI InChI=1S/C24H22F3N5/c1-31-10-12-32(13-11-31)22-9-6-18(15-28-22)16-2-4-17(5-3-16)23-29-20-8-7-19(24(25,26)27)14-21(20)30-23/h2-9,14-15H,10-13H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313976

(2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES CC(C)(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:21| Show InChI InChI=1S/C19H22N4O5/c1-19(2,18(27)21-13-6-4-3-5-12(13)17(25)26)9-15-22-16(23-28-15)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523610
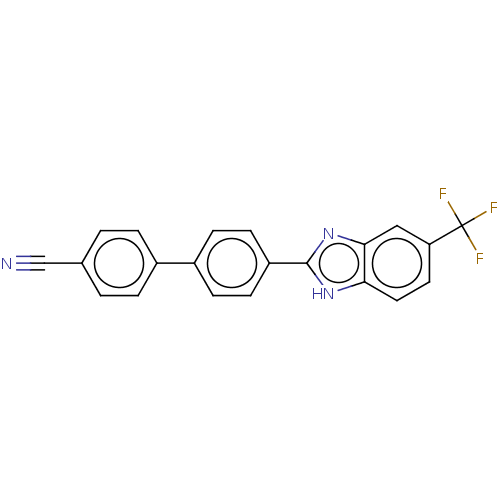
(CHEMBL4472207)Show SMILES FC(F)(F)c1ccc2[nH]c(nc2c1)-c1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H12F3N3/c22-21(23,24)17-9-10-18-19(11-17)27-20(26-18)16-7-5-15(6-8-16)14-3-1-13(12-25)2-4-14/h1-11H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 616 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313981

(CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...)Show SMILES OC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C17H18N4O6/c22-9-5-6-12(18-8-9)15-20-14(27-21-15)7-13(23)16(24)19-11-4-2-1-3-10(11)17(25)26/h5-6,8,13,22-23H,1-4,7H2,(H,19,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523611

(CHEMBL4453601)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1ccc(cc1)-c1nc2cc(ccc2[nH]1)C(F)(F)F Show InChI InChI=1S/C25H23F3N4/c1-31-12-14-32(15-13-31)21-9-6-18(7-10-21)17-2-4-19(5-3-17)24-29-22-11-8-20(25(26,27)28)16-23(22)30-24/h2-11,16H,12-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313984

(2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C(F)(F)C(F)(F)c1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H14F4N4O5/c18-16(19,14(29)23-10-4-2-1-3-9(10)13(27)28)17(20,21)15-24-12(25-30-15)11-6-5-8(26)7-22-11/h5-7,26H,1-4H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313980

(2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C1(Cc2nc(no2)-c2ccc(O)cn2)CCC1 |t:3| Show InChI InChI=1S/C20H22N4O5/c25-12-6-7-15(21-11-12)17-23-16(29-24-17)10-20(8-3-9-20)19(28)22-14-5-2-1-4-13(14)18(26)27/h6-7,11,25H,1-5,8-10H2,(H,22,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313982

(CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...)Show SMILES CC(CC(=O)NC1=C(CCCC1)C(O)=O)c1nc(no1)-c1ccc(O)cn1 |t:6| Show InChI InChI=1S/C18H20N4O5/c1-10(8-15(24)20-13-5-3-2-4-12(13)18(25)26)17-21-16(22-27-17)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523601

(CHEMBL4467163)Show SMILES OC(=O)c1ccc(Oc2ccc(cn2)-c2ccc(cn2)-c2nc3cc(Cl)ccc3[nH]2)cc1 Show InChI InChI=1S/C24H15ClN4O3/c25-17-5-9-20-21(11-17)29-23(28-20)16-3-8-19(26-13-16)15-4-10-22(27-12-15)32-18-6-1-14(2-7-18)24(30)31/h1-13H,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523622

(CHEMBL4525823)Show SMILES FC(F)(F)c1ccc2[nH]c(nc2c1)-c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C19H12F3N3/c20-19(21,22)15-7-8-16-17(10-15)25-18(24-16)13-5-3-12(4-6-13)14-2-1-9-23-11-14/h1-11H,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523624
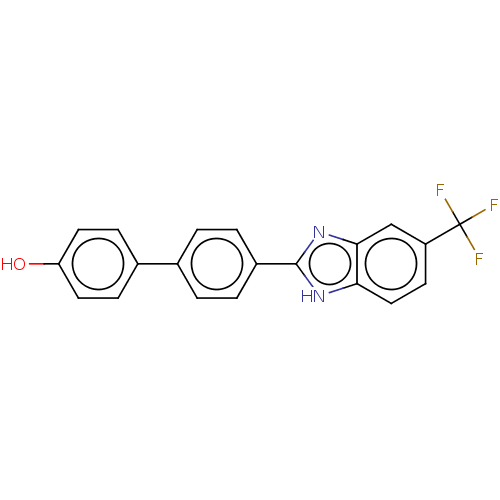
(CHEMBL4575608)Show SMILES Oc1ccc(cc1)-c1ccc(cc1)-c1nc2cc(ccc2[nH]1)C(F)(F)F Show InChI InChI=1S/C20H13F3N2O/c21-20(22,23)15-7-10-17-18(11-15)25-19(24-17)14-3-1-12(2-4-14)13-5-8-16(26)9-6-13/h1-11,26H,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523614
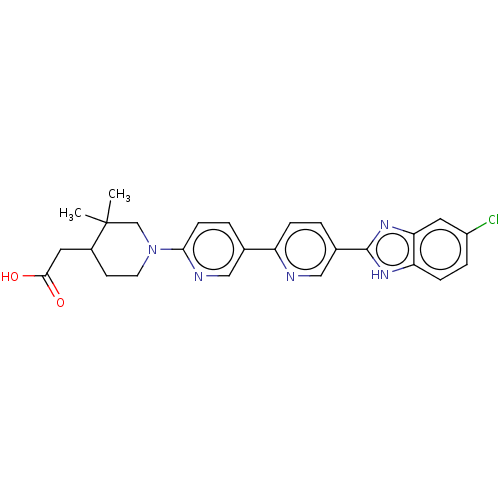
(CHEMBL4562424)Show SMILES CC1(C)CN(CCC1CC(O)=O)c1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C26H26ClN5O2/c1-26(2)15-32(10-9-18(26)11-24(33)34)23-8-4-16(13-29-23)20-6-3-17(14-28-20)25-30-21-7-5-19(27)12-22(21)31-25/h3-8,12-14,18H,9-11,15H2,1-2H3,(H,30,31)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523591
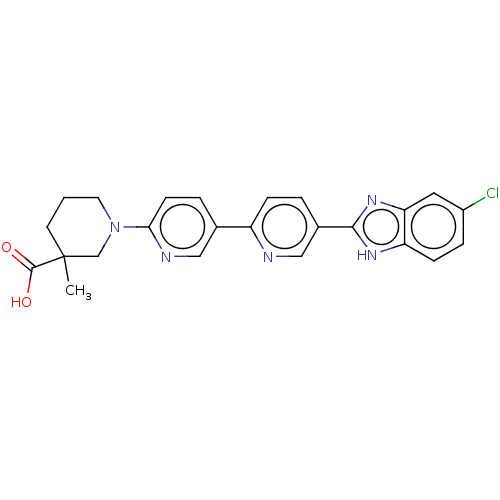
(CHEMBL4516271)Show SMILES CC1(CCCN(C1)c1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1)C(O)=O Show InChI InChI=1S/C24H22ClN5O2/c1-24(23(31)32)9-2-10-30(14-24)21-8-4-15(12-27-21)18-6-3-16(13-26-18)22-28-19-7-5-17(25)11-20(19)29-22/h3-8,11-13H,2,9-10,14H2,1H3,(H,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523623

(CHEMBL4443018)Show SMILES Oc1ccc(cn1)-c1ccc(cc1)-c1nc2cc(ccc2[nH]1)C(F)(F)F Show InChI InChI=1S/C19H12F3N3O/c20-19(21,22)14-6-7-15-16(9-14)25-18(24-15)12-3-1-11(2-4-12)13-5-8-17(26)23-10-13/h1-10H,(H,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523621
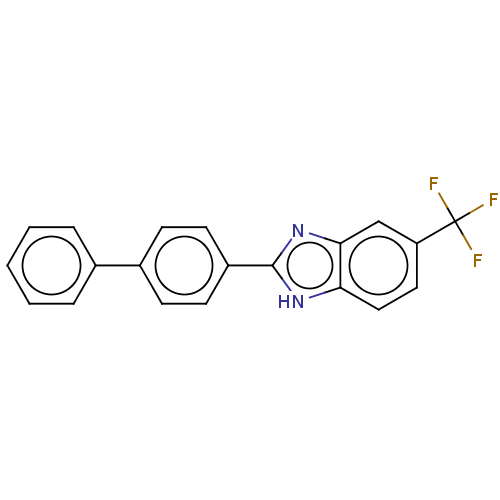
(CHEMBL4541477)Show SMILES FC(F)(F)c1ccc2[nH]c(nc2c1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C20H13F3N2/c21-20(22,23)16-10-11-17-18(12-16)25-19(24-17)15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-12H,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523597

(CHEMBL4561313)Show SMILES OC(=O)C[C@H]1CC[C@H](CC1)Oc1ccc(cn1)-c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C#N |r,wD:7.10,4.3,(71.52,-14.35,;70.76,-13.02,;69.22,-13.01,;71.53,-11.69,;70.76,-10.35,;71.53,-9.02,;70.76,-7.67,;69.23,-7.69,;68.45,-9.02,;69.22,-10.34,;68.46,-6.36,;66.92,-6.36,;66.14,-5.02,;64.6,-5.03,;63.84,-6.36,;64.61,-7.69,;66.15,-7.69,;62.3,-6.36,;61.53,-5.02,;59.99,-5.02,;59.22,-6.35,;59.99,-7.69,;61.53,-7.69,;57.69,-6.35,;56.78,-5.1,;55.31,-5.58,;53.98,-4.81,;52.65,-5.58,;52.64,-7.13,;53.98,-7.9,;55.31,-7.12,;56.78,-7.6,;51.32,-4.81,;49.98,-4.04,)| Show InChI InChI=1S/C26H23N5O3/c27-13-17-3-8-22-23(11-17)31-26(30-22)19-4-9-21(28-15-19)18-5-10-24(29-14-18)34-20-6-1-16(2-7-20)12-25(32)33/h3-5,8-11,14-16,20H,1-2,6-7,12H2,(H,30,31)(H,32,33)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523594

(CHEMBL4453443)Show SMILES OC(=O)C[C@H]1CC[C@H](CC1)Oc1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wD:7.10,4.3,(78.66,-47.58,;77.89,-46.25,;76.35,-46.24,;78.67,-44.91,;77.9,-43.58,;78.67,-42.25,;77.9,-40.91,;76.37,-40.92,;75.59,-42.25,;76.36,-43.57,;75.6,-39.59,;74.06,-39.59,;73.28,-38.25,;71.74,-38.26,;70.98,-39.59,;71.75,-40.92,;73.29,-40.92,;69.44,-39.59,;68.67,-38.25,;67.13,-38.25,;66.36,-39.58,;67.13,-40.92,;68.67,-40.92,;64.83,-39.58,;63.92,-38.33,;62.45,-38.81,;61.12,-38.04,;59.79,-38.82,;58.45,-38.05,;59.78,-40.36,;61.12,-41.13,;62.45,-40.35,;63.92,-40.83,)| Show InChI InChI=1S/C25H23ClN4O3/c26-18-5-9-21-22(12-18)30-25(29-21)17-3-8-20(27-14-17)16-4-10-23(28-13-16)33-19-6-1-15(2-7-19)11-24(31)32/h3-5,8-10,12-15,19H,1-2,6-7,11H2,(H,29,30)(H,31,32)/t15-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523620
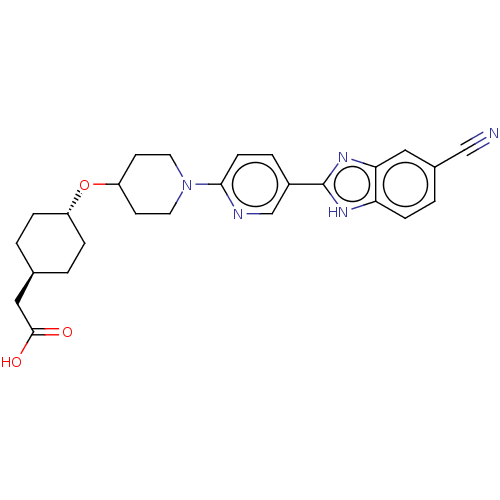
(CHEMBL4452750)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C#N |r,wU:7.10,wD:4.3,(72.91,-13.35,;72.15,-12.01,;70.61,-11.99,;72.93,-10.68,;72.17,-9.34,;72.95,-8.01,;72.19,-6.67,;70.65,-6.67,;69.87,-7.99,;70.63,-9.33,;69.89,-5.34,;68.35,-5.33,;67.57,-6.65,;66.04,-6.65,;65.29,-5.31,;66.04,-3.99,;67.59,-3.99,;63.75,-5.31,;62.97,-3.97,;61.44,-3.98,;60.67,-5.31,;61.44,-6.64,;62.98,-6.65,;59.14,-5.31,;58.23,-4.06,;56.76,-4.53,;55.42,-3.77,;54.09,-4.54,;54.09,-6.09,;55.43,-6.86,;56.76,-6.08,;58.23,-6.56,;52.76,-3.77,;51.43,-3,)| Show InChI InChI=1S/C26H29N5O3/c27-15-18-3-7-22-23(13-18)30-26(29-22)19-4-8-24(28-16-19)31-11-9-21(10-12-31)34-20-5-1-17(2-6-20)14-25(32)33/h3-4,7-8,13,16-17,20-21H,1-2,5-6,9-12,14H2,(H,29,30)(H,32,33)/t17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031501
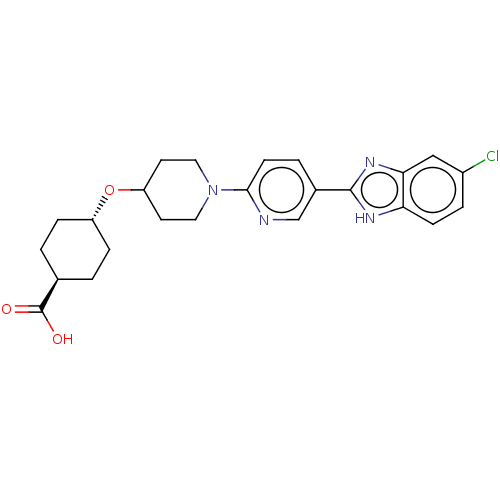
(CHEMBL3342773)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wU:6.9,wD:3.2,(37.68,-12.65,;37.68,-11.11,;39.02,-10.34,;36.35,-10.33,;36.35,-8.79,;35.02,-8.01,;33.69,-8.77,;33.68,-10.32,;35.01,-11.09,;32.35,-8,;31.02,-8.77,;31.02,-10.31,;29.69,-11.08,;28.36,-10.31,;28.35,-8.78,;29.68,-8,;27.02,-11.08,;25.69,-10.32,;24.36,-11.09,;24.36,-12.64,;25.69,-13.41,;27.03,-12.63,;23.02,-13.4,;21.62,-12.77,;20.59,-13.92,;19.05,-13.92,;18.29,-15.25,;16.75,-15.25,;19.06,-16.58,;20.59,-16.57,;21.35,-15.25,;22.86,-14.93,)| Show InChI InChI=1S/C24H27ClN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50437390

(CHEMBL2408633)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)Oc1ccc(cn1)-c1ccc(cn1)-c1nc2ccc(Cl)cc2[nH]1 |r,wU:6.9,wD:3.2,(43.88,-64.06,;44.65,-62.73,;46.19,-62.73,;43.87,-61.38,;44.64,-60.05,;43.87,-58.71,;42.34,-58.73,;41.57,-60.05,;42.33,-61.38,;41.57,-57.4,;40.03,-57.4,;39.25,-56.06,;37.71,-56.07,;36.95,-57.4,;37.72,-58.74,;39.26,-58.74,;35.41,-57.4,;34.63,-56.06,;33.1,-56.07,;32.33,-57.4,;33.1,-58.74,;34.64,-58.74,;30.79,-57.4,;29.89,-58.65,;28.42,-58.17,;27.09,-58.93,;25.76,-58.17,;25.76,-56.63,;24.43,-55.86,;27.09,-55.85,;28.42,-56.63,;29.89,-56.15,)| Show InChI InChI=1S/C24H21ClN4O3/c25-17-5-9-20-21(11-17)29-23(28-20)16-3-8-19(26-13-16)15-4-10-22(27-12-15)32-18-6-1-14(2-7-18)24(30)31/h3-5,8-14,18H,1-2,6-7H2,(H,28,29)(H,30,31)/t14-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523625
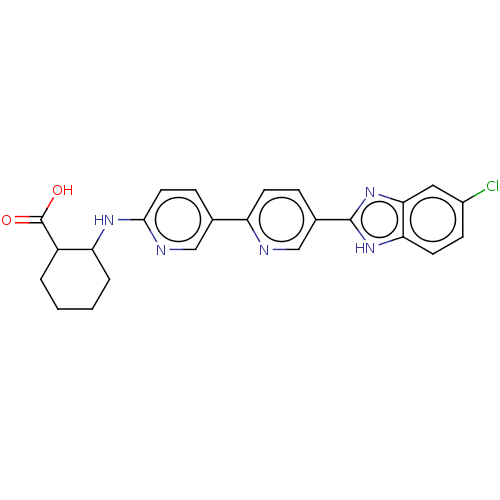
(CHEMBL4554202)Show SMILES OC(=O)C1CCCCC1Nc1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C24H22ClN5O2/c25-16-7-9-20-21(11-16)30-23(29-20)15-5-8-18(26-13-15)14-6-10-22(27-12-14)28-19-4-2-1-3-17(19)24(31)32/h5-13,17,19H,1-4H2,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523615
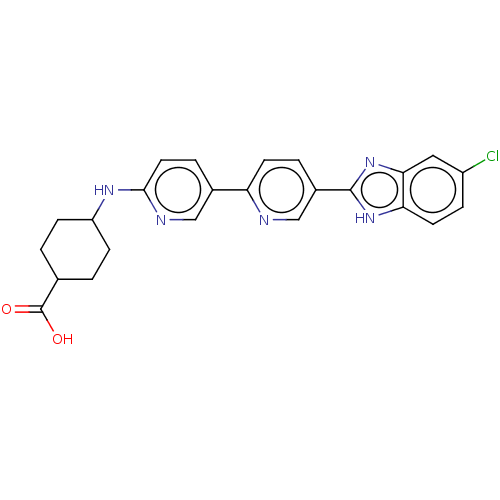
(CHEMBL4461413)Show SMILES OC(=O)C1CCC(CC1)Nc1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |(53.38,-45.19,;51.84,-45.2,;51.06,-43.87,;51.08,-46.54,;51.85,-47.87,;51.09,-49.2,;49.56,-49.2,;48.77,-47.89,;49.54,-46.55,;48.8,-50.54,;47.26,-50.56,;46.47,-49.23,;44.94,-49.24,;44.18,-50.58,;44.96,-51.91,;46.5,-51.9,;42.65,-50.59,;41.87,-49.26,;40.33,-49.27,;39.57,-50.61,;40.35,-51.94,;41.89,-51.93,;38.04,-50.62,;37.12,-49.37,;35.66,-49.86,;34.32,-49.1,;32.99,-49.87,;31.66,-49.1,;32.99,-51.41,;34.33,-52.18,;35.67,-51.4,;37.14,-51.87,)| Show InChI InChI=1S/C24H22ClN5O2/c25-17-5-9-20-21(11-17)30-23(29-20)16-3-8-19(26-13-16)15-4-10-22(27-12-15)28-18-6-1-14(2-7-18)24(31)32/h3-5,8-14,18H,1-2,6-7H2,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523604
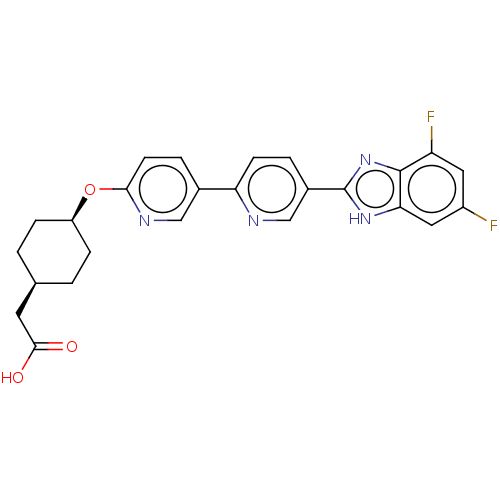
(CHEMBL4458847)Show SMILES OC(=O)C[C@H]1CC[C@H](CC1)Oc1ccc(cn1)-c1ccc(cn1)-c1nc2c(F)cc(F)cc2[nH]1 |r,wD:7.10,4.3,(77.9,-58.59,;77.14,-57.26,;75.6,-57.25,;77.91,-55.92,;77.14,-54.59,;77.91,-53.25,;77.15,-51.91,;75.61,-51.93,;74.84,-53.25,;75.6,-54.58,;74.84,-50.59,;73.3,-50.6,;72.52,-49.26,;70.99,-49.27,;70.22,-50.59,;70.99,-51.93,;72.53,-51.93,;68.68,-50.59,;67.91,-49.25,;66.37,-49.26,;65.61,-50.59,;66.37,-51.93,;67.91,-51.93,;64.07,-50.59,;63.16,-49.34,;61.69,-49.81,;60.36,-49.05,;60.35,-47.51,;59.03,-49.82,;59.03,-51.37,;57.69,-52.14,;60.36,-52.14,;61.69,-51.36,;63.16,-51.84,)| Show InChI InChI=1S/C25H22F2N4O3/c26-17-10-19(27)24-21(11-17)30-25(31-24)16-3-7-20(28-13-16)15-4-8-22(29-12-15)34-18-5-1-14(2-6-18)9-23(32)33/h3-4,7-8,10-14,18H,1-2,5-6,9H2,(H,30,31)(H,32,33)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031501

(CHEMBL3342773)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wU:6.9,wD:3.2,(37.68,-12.65,;37.68,-11.11,;39.02,-10.34,;36.35,-10.33,;36.35,-8.79,;35.02,-8.01,;33.69,-8.77,;33.68,-10.32,;35.01,-11.09,;32.35,-8,;31.02,-8.77,;31.02,-10.31,;29.69,-11.08,;28.36,-10.31,;28.35,-8.78,;29.68,-8,;27.02,-11.08,;25.69,-10.32,;24.36,-11.09,;24.36,-12.64,;25.69,-13.41,;27.03,-12.63,;23.02,-13.4,;21.62,-12.77,;20.59,-13.92,;19.05,-13.92,;18.29,-15.25,;16.75,-15.25,;19.06,-16.58,;20.59,-16.57,;21.35,-15.25,;22.86,-14.93,)| Show InChI InChI=1S/C24H27ClN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523584

(CHEMBL4452940)Show SMILES OC(=O)C[C@H]1CC[C@H](CC1)Oc1ccc(cn1)-c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wD:7.10,4.3,(24.82,-57.8,;24.05,-56.46,;22.51,-56.46,;24.82,-55.13,;24.05,-53.8,;24.83,-52.46,;24.06,-51.12,;22.52,-51.14,;21.75,-52.46,;22.51,-53.79,;21.75,-49.8,;20.21,-49.81,;19.43,-48.47,;17.9,-48.47,;17.13,-49.8,;17.9,-51.14,;19.44,-51.14,;15.6,-49.8,;14.82,-48.46,;13.28,-48.47,;12.52,-49.8,;13.28,-51.13,;14.82,-51.14,;10.98,-49.8,;10.08,-48.55,;8.6,-49.02,;7.27,-48.26,;5.94,-49.03,;5.94,-50.58,;7.27,-51.35,;8.6,-50.57,;10.07,-51.05,;4.61,-48.26,;4.61,-46.72,;3.27,-49.03,;3.27,-47.49,)| Show InChI InChI=1S/C26H23F3N4O3/c27-26(28,29)18-5-9-21-22(12-18)33-25(32-21)17-3-8-20(30-14-17)16-4-10-23(31-13-16)36-19-6-1-15(2-7-19)11-24(34)35/h3-5,8-10,12-15,19H,1-2,6-7,11H2,(H,32,33)(H,34,35)/t15-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523600
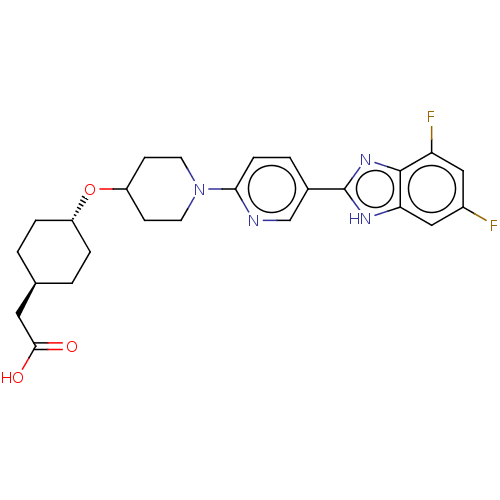
(CHEMBL4464262)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2c(F)cc(F)cc2[nH]1 |r,wU:7.10,wD:4.3,(22.62,-56.01,;21.86,-54.67,;20.32,-54.66,;22.64,-53.34,;21.88,-52,;22.66,-50.67,;21.9,-49.33,;20.37,-49.33,;19.59,-50.65,;20.34,-51.99,;19.61,-48,;18.07,-47.99,;17.29,-49.32,;15.76,-49.31,;15,-47.98,;15.76,-46.65,;17.3,-46.65,;13.46,-47.97,;12.69,-46.64,;11.15,-46.64,;10.39,-47.97,;11.15,-49.31,;12.69,-49.31,;8.85,-47.97,;7.94,-46.72,;6.47,-47.19,;5.14,-46.43,;5.13,-44.89,;3.81,-47.2,;3.81,-48.75,;2.47,-49.52,;5.14,-49.52,;6.47,-48.74,;7.94,-49.22,)| Show InChI InChI=1S/C25H28F2N4O3/c26-17-12-20(27)24-21(13-17)29-25(30-24)16-3-6-22(28-14-16)31-9-7-19(8-10-31)34-18-4-1-15(2-5-18)11-23(32)33/h3,6,12-15,18-19H,1-2,4-5,7-11H2,(H,29,30)(H,32,33)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523596

(CHEMBL4473500)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wU:7.10,wD:4.3,(73.88,-44.52,;73.12,-43.18,;71.58,-43.17,;73.9,-41.85,;73.14,-40.51,;73.92,-39.18,;73.16,-37.84,;71.63,-37.85,;70.84,-39.17,;71.6,-40.5,;70.86,-36.51,;69.32,-36.5,;68.55,-37.83,;67.02,-37.82,;66.26,-36.49,;67.02,-35.16,;68.56,-35.17,;64.72,-36.49,;63.94,-35.15,;62.41,-35.15,;61.64,-36.48,;62.41,-37.82,;63.95,-37.82,;60.11,-36.48,;59.2,-35.23,;57.73,-35.71,;56.39,-34.95,;55.07,-35.72,;53.73,-34.95,;55.06,-37.26,;56.4,-38.03,;57.73,-37.25,;59.2,-37.73,)| Show InChI InChI=1S/C25H29ClN4O3/c26-18-4-7-21-22(14-18)29-25(28-21)17-3-8-23(27-15-17)30-11-9-20(10-12-30)33-19-5-1-16(2-6-19)13-24(31)32/h3-4,7-8,14-16,19-20H,1-2,5-6,9-13H2,(H,28,29)(H,31,32)/t16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031503

(CHEMBL3342775)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wU:6.9,wD:3.2,(29.05,-9.85,;29.05,-8.31,;30.39,-7.55,;27.72,-7.54,;27.72,-6,;26.39,-5.22,;25.06,-5.98,;25.06,-7.53,;26.38,-8.3,;23.73,-5.21,;22.39,-5.98,;22.39,-7.52,;21.07,-8.29,;19.73,-7.52,;19.72,-5.98,;21.06,-5.21,;18.4,-8.29,;17.06,-7.53,;15.73,-8.3,;15.73,-9.84,;17.06,-10.61,;18.4,-9.84,;14.4,-10.61,;12.99,-9.98,;11.96,-11.13,;10.43,-11.13,;9.66,-12.46,;10.44,-13.79,;11.97,-13.78,;12.73,-12.46,;14.23,-12.14,;8.12,-12.46,;7.35,-11.13,;7.35,-13.79,;6.58,-12.45,)| Show InChI InChI=1S/C25H27F3N4O3/c26-25(27,28)17-4-7-20-21(13-17)31-23(30-20)16-3-8-22(29-14-16)32-11-9-19(10-12-32)35-18-5-1-15(2-6-18)24(33)34/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,30,31)(H,33,34)/t15-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523612

(CHEMBL4476596)Show SMILES CC1(CCN(CC1)c1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1)C(O)=O Show InChI InChI=1S/C24H22ClN5O2/c1-24(23(31)32)8-10-30(11-9-24)21-7-3-15(13-27-21)18-5-2-16(14-26-18)22-28-19-6-4-17(25)12-20(19)29-22/h2-7,12-14H,8-11H2,1H3,(H,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523580

(CHEMBL4572398)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wU:7.10,wD:4.3,(48.94,-42.6,;48.18,-41.26,;46.64,-41.25,;48.96,-39.93,;48.2,-38.59,;48.98,-37.26,;48.22,-35.92,;46.69,-35.92,;45.91,-37.24,;46.66,-38.58,;45.92,-34.59,;44.38,-34.58,;43.61,-35.91,;42.08,-35.9,;41.32,-34.57,;42.08,-33.24,;43.62,-33.25,;39.78,-34.56,;39.01,-33.23,;37.47,-33.23,;36.7,-34.56,;37.47,-35.9,;39.01,-35.9,;35.17,-34.56,;34.26,-33.31,;32.79,-33.78,;31.46,-33.02,;30.13,-33.79,;30.13,-35.34,;31.46,-36.11,;32.79,-35.33,;34.26,-35.81,;28.79,-33.02,;28.79,-31.48,;27.46,-33.79,;27.45,-32.25,)| Show InChI InChI=1S/C26H29F3N4O3/c27-26(28,29)18-4-7-21-22(14-18)32-25(31-21)17-3-8-23(30-15-17)33-11-9-20(10-12-33)36-19-5-1-16(2-6-19)13-24(34)35/h3-4,7-8,14-16,19-20H,1-2,5-6,9-13H2,(H,31,32)(H,34,35)/t16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523626
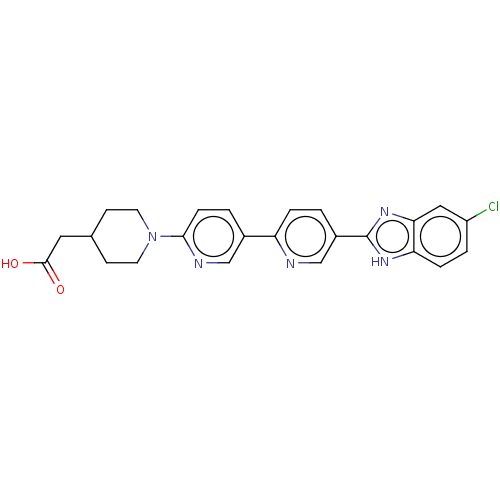
(CHEMBL4450287)Show SMILES OC(=O)CC1CCN(CC1)c1ccc(cn1)-c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C24H22ClN5O2/c25-18-3-5-20-21(12-18)29-24(28-20)17-1-4-19(26-14-17)16-2-6-22(27-13-16)30-9-7-15(8-10-30)11-23(31)32/h1-6,12-15H,7-11H2,(H,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50437391
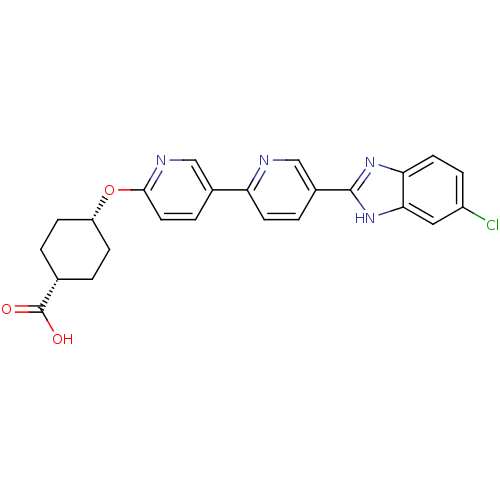
(CHEMBL2408632)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)Oc1ccc(cn1)-c1ccc(cn1)-c1nc2ccc(Cl)cc2[nH]1 |r,wU:6.9,3.2,(43.88,-64.06,;44.65,-62.73,;46.19,-62.73,;43.87,-61.38,;44.64,-60.05,;43.87,-58.71,;42.34,-58.73,;41.57,-60.05,;42.33,-61.38,;41.57,-57.4,;40.03,-57.4,;39.25,-56.06,;37.71,-56.07,;36.95,-57.4,;37.72,-58.74,;39.26,-58.74,;35.41,-57.4,;34.63,-56.06,;33.1,-56.07,;32.33,-57.4,;33.1,-58.74,;34.64,-58.74,;30.79,-57.4,;29.89,-58.65,;28.42,-58.17,;27.09,-58.93,;25.76,-58.17,;25.76,-56.63,;24.43,-55.86,;27.09,-55.85,;28.42,-56.63,;29.89,-56.15,)| Show InChI InChI=1S/C24H21ClN4O3/c25-17-5-9-20-21(11-17)29-23(28-20)16-3-8-19(26-13-16)15-4-10-22(27-12-15)32-18-6-1-14(2-7-18)24(30)31/h3-5,8-14,18H,1-2,6-7H2,(H,28,29)(H,30,31)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50523618

(CHEMBL4527223)Show SMILES OC(=O)C1(COc2ccc(cn2)-c2ccc(cn2)-c2nc3cc(Cl)ccc3[nH]2)COC1 Show InChI InChI=1S/C22H17ClN4O4/c23-15-3-5-17-18(7-15)27-20(26-17)14-1-4-16(24-9-14)13-2-6-19(25-8-13)31-12-22(21(28)29)10-30-11-22/h1-9H,10-12H2,(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data