 Found 35 hits with Last Name = 'wong' and Initial = 'kr'
Found 35 hits with Last Name = 'wong' and Initial = 'kr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25743

(1-cycloheptyl-3-(1-acetylpiperidin-4-yl)urea | US8...)Show InChI InChI=1S/C15H27N3O2/c1-12(19)18-10-8-14(9-11-18)17-15(20)16-13-6-4-2-3-5-7-13/h13-14H,2-11H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335964

(1-(1-nicotinoylpiperidin-4-yl)-3-(4-(trifluorometh...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H19F3N4O3/c20-19(21,22)29-16-5-3-14(4-6-16)24-18(28)25-15-7-10-26(11-8-15)17(27)13-2-1-9-23-12-13/h1-6,9,12,15H,7-8,10-11H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335965
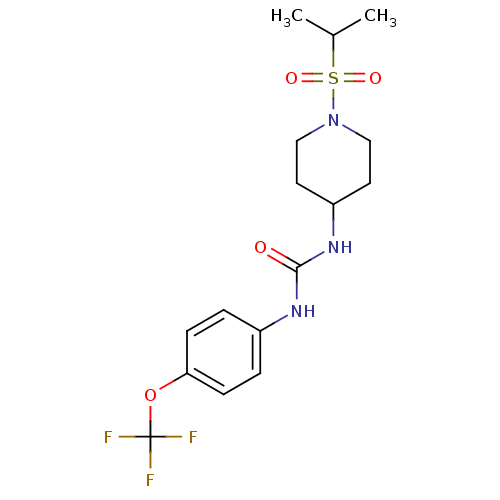
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H22F3N3O4S/c1-11(2)27(24,25)22-9-7-13(8-10-22)21-15(23)20-12-3-5-14(6-4-12)26-16(17,18)19/h3-6,11,13H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966
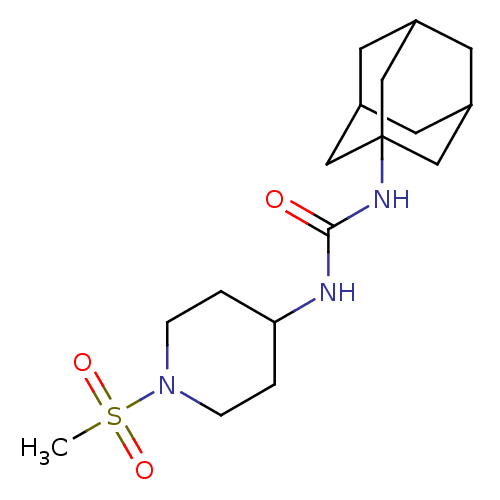
(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335967
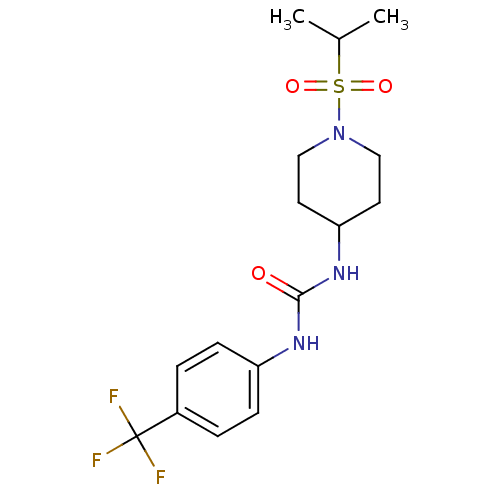
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H22F3N3O3S/c1-11(2)26(24,25)22-9-7-14(8-10-22)21-15(23)20-13-5-3-12(4-6-13)16(17,18)19/h3-6,11,14H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50191854
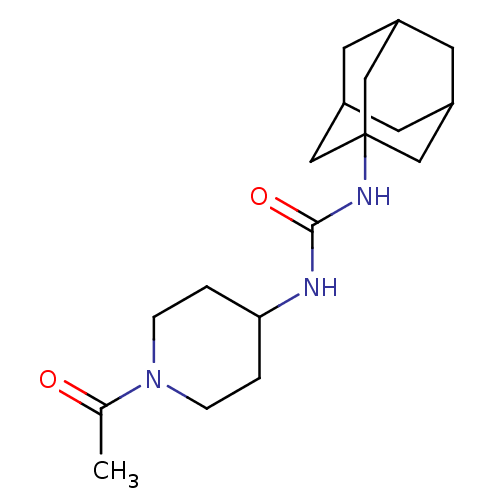
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335968
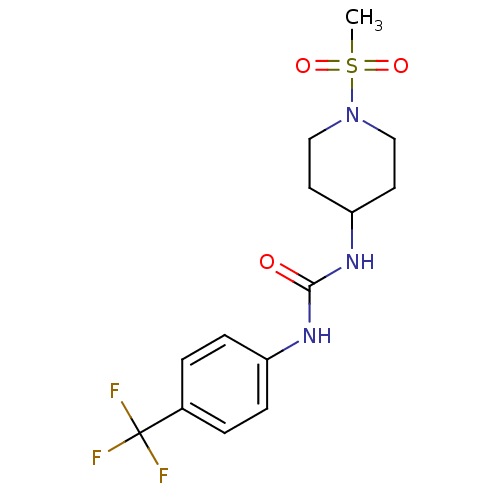
(1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H18F3N3O3S/c1-24(22,23)20-8-6-12(7-9-20)19-13(21)18-11-4-2-10(3-5-11)14(15,16)17/h2-5,12H,6-9H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966

(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335969

(1-(1-acetylpiperidin-4-yl)-3-(4,4-dimethylcyclohex...)Show InChI InChI=1S/C16H29N3O2/c1-12(20)19-10-6-14(7-11-19)18-15(21)17-13-4-8-16(2,3)9-5-13/h13-14H,4-11H2,1-3H3,(H2,17,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335970

(1-cyclohexyl-3-(1-picolinoylpiperidin-4-yl)urea | ...)Show InChI InChI=1S/C18H26N4O2/c23-17(16-8-4-5-11-19-16)22-12-9-15(10-13-22)21-18(24)20-14-6-2-1-3-7-14/h4-5,8,11,14-15H,1-3,6-7,9-10,12-13H2,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335964

(1-(1-nicotinoylpiperidin-4-yl)-3-(4-(trifluorometh...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H19F3N4O3/c20-19(21,22)29-16-5-3-14(4-6-16)24-18(28)25-15-7-10-26(11-8-15)17(27)13-2-1-9-23-12-13/h1-6,9,12,15H,7-8,10-11H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854

(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335971
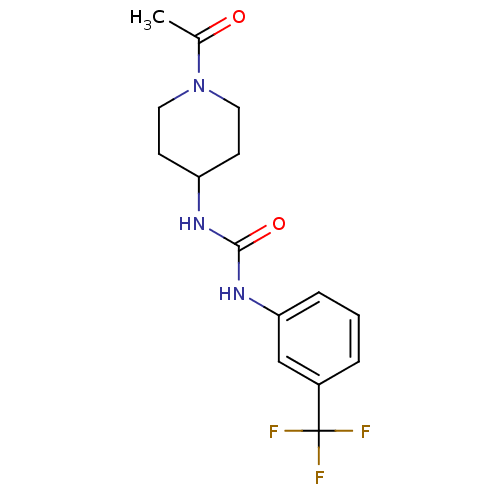
(1-(1-acetylpiperidin-4-yl)-3-(3-(trifluoromethyl)p...)Show InChI InChI=1S/C15H18F3N3O2/c1-10(22)21-7-5-12(6-8-21)19-14(23)20-13-4-2-3-11(9-13)15(16,17)18/h2-4,9,12H,5-8H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335972
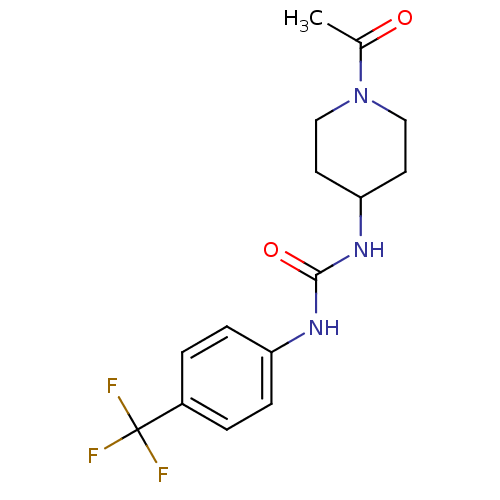
(1-(1-acetylpiperidin-4-yl)-3-(4-(trifluoromethyl)p...)Show InChI InChI=1S/C15H18F3N3O2/c1-10(22)21-8-6-13(7-9-21)20-14(23)19-12-4-2-11(3-5-12)15(16,17)18/h2-5,13H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25744
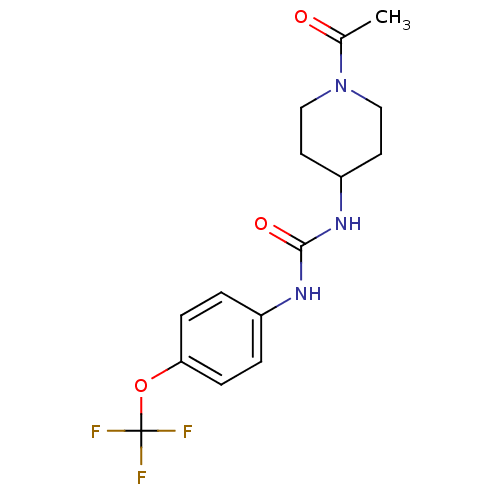
(3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...)Show InChI InChI=1S/C15H18F3N3O3/c1-10(22)21-8-6-12(7-9-21)20-14(23)19-11-2-4-13(5-3-11)24-15(16,17)18/h2-5,12H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335973

(1-(1-acetylpiperidin-4-yl)-3-(4-tert-butylcyclohex...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC1CCC(CC1)C(C)(C)C |(1.63,-5.6,;1.57,-7.14,;.21,-7.87,;2.88,-7.96,;4.25,-7.24,;5.55,-8.05,;5.49,-9.6,;4.14,-10.32,;2.83,-9.51,;6.8,-10.41,;6.75,-11.95,;5.39,-12.68,;8.07,-12.76,;8.05,-14.31,;6.71,-15.06,;6.69,-16.59,;8.02,-17.38,;9.36,-16.62,;9.38,-15.08,;8,-18.92,;6.66,-19.67,;9.32,-19.7,;7.98,-20.45,)| Show InChI InChI=1S/C18H33N3O2/c1-13(22)21-11-9-16(10-12-21)20-17(23)19-15-7-5-14(6-8-15)18(2,3)4/h14-16H,5-12H2,1-4H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335967

(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H22F3N3O3S/c1-11(2)26(24,25)22-9-7-14(8-10-22)21-15(23)20-13-5-3-12(4-6-13)16(17,18)19/h3-6,11,14H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335974
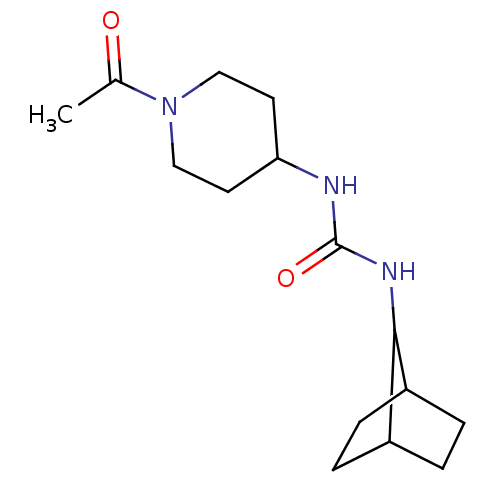
(1-(1-Acetyl-piperidin-4-yl)-3-bicyclo[2.2.1]hept-7...)Show InChI InChI=1S/C15H25N3O2/c1-10(19)18-8-6-13(7-9-18)16-15(20)17-14-11-2-3-12(14)5-4-11/h11-14H,2-9H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335975

(1-(1-Acetyl-piperidin-4-yl)-3-(4-methyl-bicyclo[2....)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC1CC2(C)CCC1CC2 |(19.45,7.01,;19.4,5.47,;18.03,4.74,;20.7,4.65,;22.06,5.37,;23.37,4.56,;23.3,3.02,;21.96,2.29,;20.65,3.11,;24.61,2.21,;24.57,.67,;23.21,-.06,;25.88,-.14,;25.86,-1.68,;26.33,-2.77,;26.12,-4.67,;26.14,-6.19,;27.5,-5.27,;27.19,-3.88,;25.83,-3.31,;24.39,-3.99,;24.61,-5.36,)| Show InChI InChI=1S/C17H29N3O2/c1-12(21)20-9-5-14(6-10-20)18-16(22)19-15-11-17(2)7-3-13(15)4-8-17/h13-15H,3-11H2,1-2H3,(H2,18,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335968

(1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H18F3N3O3S/c1-24(22,23)20-8-6-12(7-9-20)19-13(21)18-11-4-2-10(3-5-11)14(15,16)17/h2-5,12H,6-9H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25743

(1-cycloheptyl-3-(1-acetylpiperidin-4-yl)urea | US8...)Show InChI InChI=1S/C15H27N3O2/c1-12(19)18-10-8-14(9-11-18)17-15(20)16-13-6-4-2-3-5-7-13/h13-14H,2-11H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335976
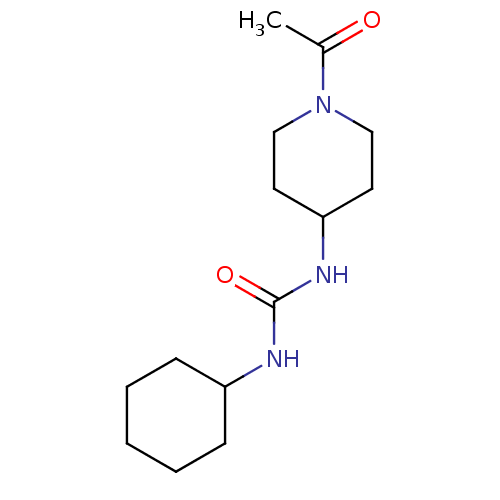
(1-(1-Acetyl-piperidin-4-yl)-3-cyclohexyl-urea | CH...)Show InChI InChI=1S/C14H25N3O2/c1-11(18)17-9-7-13(8-10-17)16-14(19)15-12-5-3-2-4-6-12/h12-13H,2-10H2,1H3,(H2,15,16,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335969

(1-(1-acetylpiperidin-4-yl)-3-(4,4-dimethylcyclohex...)Show InChI InChI=1S/C16H29N3O2/c1-12(20)19-10-6-14(7-11-19)18-15(21)17-13-4-8-16(2,3)9-5-13/h13-14H,4-11H2,1-3H3,(H2,17,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854

(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335977

(1-(1-Acetyl-piperidin-4-yl)-3-(3-fluoro-adamantan-...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(F)(CC(F)(C3)C1)C2 |TLB:16:17:23:22.15.14,18:17:23:22.15.14,18:17:20.22.23:14,21:20:17.16.24:14,THB:16:15:19.17.24:23,19:20:17.16.24:14,19:17:20.22.23:14| Show InChI InChI=1S/C18H27F2N3O2/c1-12(24)23-4-2-14(3-5-23)21-15(25)22-18-8-13-6-16(19,10-18)9-17(20,7-13)11-18/h13-14H,2-11H2,1H3,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25744

(3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...)Show InChI InChI=1S/C15H18F3N3O3/c1-10(22)21-8-6-12(7-9-21)20-14(23)19-11-2-4-13(5-3-11)24-15(16,17)18/h2-5,12H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335965

(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H22F3N3O4S/c1-11(2)27(24,25)22-9-7-13(8-10-22)21-15(23)20-12-3-5-14(6-4-12)26-16(17,18)19/h3-6,11,13H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335970

(1-cyclohexyl-3-(1-picolinoylpiperidin-4-yl)urea | ...)Show InChI InChI=1S/C18H26N4O2/c23-17(16-8-4-5-11-19-16)22-12-9-15(10-13-22)21-18(24)20-14-6-2-1-3-7-14/h4-5,8,11,14-15H,1-3,6-7,9-10,12-13H2,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335972

(1-(1-acetylpiperidin-4-yl)-3-(4-(trifluoromethyl)p...)Show InChI InChI=1S/C15H18F3N3O2/c1-10(22)21-8-6-13(7-9-21)20-14(23)19-12-4-2-11(3-5-12)15(16,17)18/h2-5,13H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335978

(1-(1-Acetyl-piperidin-4-yl)-3-(3,5,7-trifluoro-ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3(F)CC(F)(CC(F)(C3)C1)C2 |TLB:17:18:23.15.14:24,23:21:15.17.14:25,23:15:21.20.24:25,THB:17:15:24:20.18.25,16:15:24:20.18.25,16:15:21.20.24:25,22:21:15.17.14:25,19:18:23.15.14:24| Show InChI InChI=1S/C18H26F3N3O2/c1-12(25)24-4-2-13(3-5-24)22-14(26)23-18-9-15(19)6-16(20,10-18)8-17(21,7-15)11-18/h13H,2-11H2,1H3,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335974

(1-(1-Acetyl-piperidin-4-yl)-3-bicyclo[2.2.1]hept-7...)Show InChI InChI=1S/C15H25N3O2/c1-10(19)18-8-6-13(7-9-18)16-15(20)17-14-11-2-3-12(14)5-4-11/h11-14H,2-9H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335979
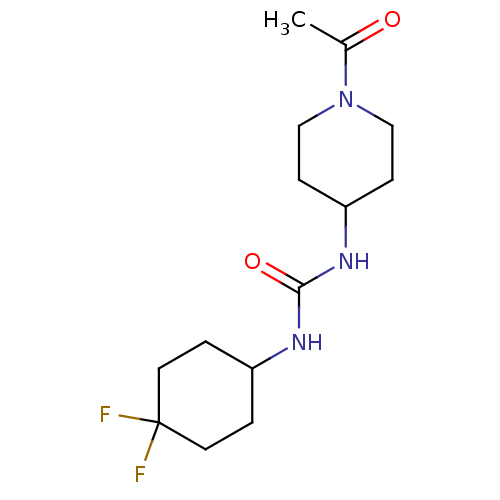
(1-(1-acetylpiperidin-4-yl)-3-(4,4-difluorocyclohex...)Show InChI InChI=1S/C14H23F2N3O2/c1-10(20)19-8-4-12(5-9-19)18-13(21)17-11-2-6-14(15,16)7-3-11/h11-12H,2-9H2,1H3,(H2,17,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335971

(1-(1-acetylpiperidin-4-yl)-3-(3-(trifluoromethyl)p...)Show InChI InChI=1S/C15H18F3N3O2/c1-10(22)21-7-5-12(6-8-21)19-14(23)20-13-4-2-3-11(9-13)15(16,17)18/h2-4,9,12H,5-8H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335973

(1-(1-acetylpiperidin-4-yl)-3-(4-tert-butylcyclohex...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC1CCC(CC1)C(C)(C)C |(1.63,-5.6,;1.57,-7.14,;.21,-7.87,;2.88,-7.96,;4.25,-7.24,;5.55,-8.05,;5.49,-9.6,;4.14,-10.32,;2.83,-9.51,;6.8,-10.41,;6.75,-11.95,;5.39,-12.68,;8.07,-12.76,;8.05,-14.31,;6.71,-15.06,;6.69,-16.59,;8.02,-17.38,;9.36,-16.62,;9.38,-15.08,;8,-18.92,;6.66,-19.67,;9.32,-19.7,;7.98,-20.45,)| Show InChI InChI=1S/C18H33N3O2/c1-13(22)21-11-9-16(10-12-21)20-17(23)19-15-7-5-14(6-8-15)18(2,3)4/h14-16H,5-12H2,1-4H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335975

(1-(1-Acetyl-piperidin-4-yl)-3-(4-methyl-bicyclo[2....)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC1CC2(C)CCC1CC2 |(19.45,7.01,;19.4,5.47,;18.03,4.74,;20.7,4.65,;22.06,5.37,;23.37,4.56,;23.3,3.02,;21.96,2.29,;20.65,3.11,;24.61,2.21,;24.57,.67,;23.21,-.06,;25.88,-.14,;25.86,-1.68,;26.33,-2.77,;26.12,-4.67,;26.14,-6.19,;27.5,-5.27,;27.19,-3.88,;25.83,-3.31,;24.39,-3.99,;24.61,-5.36,)| Show InChI InChI=1S/C17H29N3O2/c1-12(21)20-9-5-14(6-10-20)18-16(22)19-15-11-17(2)7-3-13(15)4-8-17/h13-15H,3-11H2,1-2H3,(H2,18,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data