 Found 439 hits with Last Name = 'wong' and Initial = 'tw'
Found 439 hits with Last Name = 'wong' and Initial = 'tw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50235550

(1-(4-(5-((4-aminocyclohexylidene)methyl)pyrrolo[1,...)Show SMILES NC1CCC(CC1)=Cc1ccn2ncnc(Oc3ccc(NC(=O)NC(=O)Cc4ccc(F)cc4)cc3F)c12 Show InChI InChI=1S/C28H26F2N6O3/c29-20-5-1-18(2-6-20)14-25(37)35-28(38)34-22-9-10-24(23(30)15-22)39-27-26-19(11-12-36(26)33-16-32-27)13-17-3-7-21(31)8-4-17/h1-2,5-6,9-13,15-16,21H,3-4,7-8,14,31H2,(H2,34,35,37,38)/b17-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Met expressed in insect cell-baculovirus expression system |
Bioorg Med Chem Lett 18: 1945-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.121
BindingDB Entry DOI: 10.7270/Q2125SFV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333374
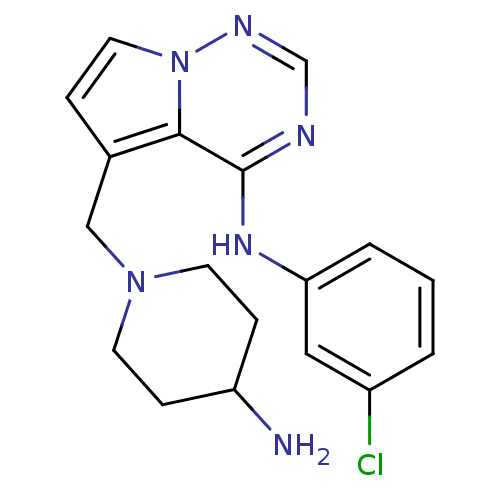
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-chloropheny...)Show InChI InChI=1S/C18H21ClN6/c19-14-2-1-3-16(10-14)23-18-17-13(4-9-25(17)22-12-21-18)11-24-7-5-15(20)6-8-24/h1-4,9-10,12,15H,5-8,11,20H2,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333375
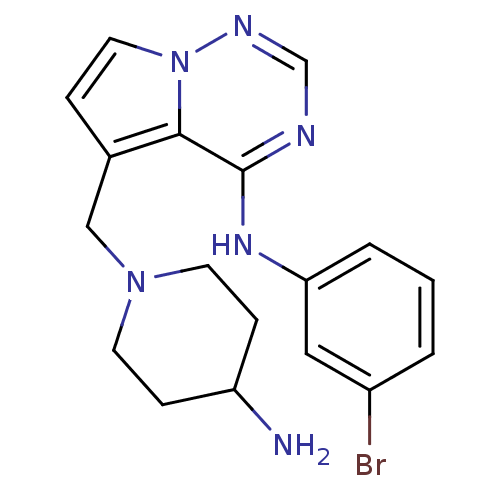
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-bromophenyl...)Show InChI InChI=1S/C18H21BrN6/c19-14-2-1-3-16(10-14)23-18-17-13(4-9-25(17)22-12-21-18)11-24-7-5-15(20)6-8-24/h1-4,9-10,12,15H,5-8,11,20H2,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333376
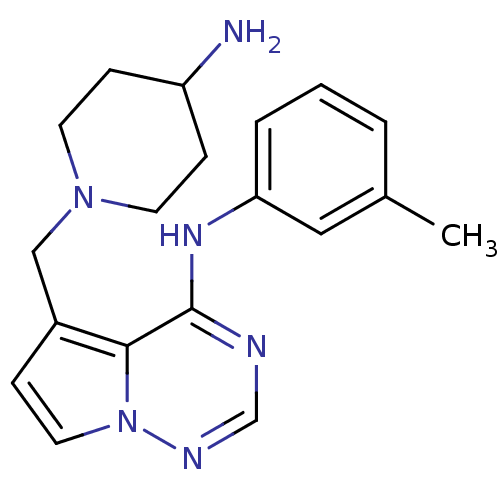
(5-((4-aminopiperidin-1-yl)methyl)-N-m-tolylpyrrolo...)Show InChI InChI=1S/C19H24N6/c1-14-3-2-4-17(11-14)23-19-18-15(5-10-25(18)22-13-21-19)12-24-8-6-16(20)7-9-24/h2-5,10-11,13,16H,6-9,12,20H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50333375

(5-((4-aminopiperidin-1-yl)methyl)-N-(3-bromophenyl...)Show InChI InChI=1S/C18H21BrN6/c19-14-2-1-3-16(10-14)23-18-17-13(4-9-25(17)22-12-21-18)11-24-7-5-15(20)6-8-24/h1-4,9-10,12,15H,5-8,11,20H2,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333377
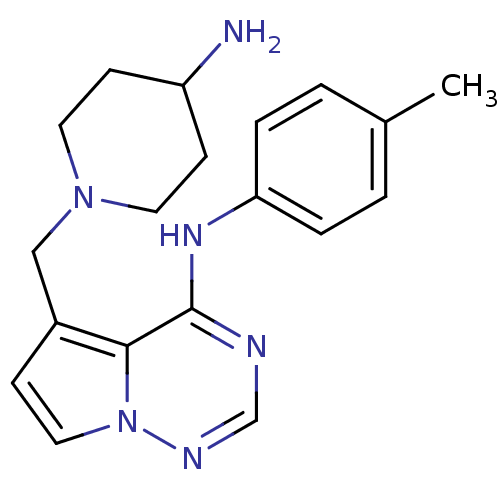
(5-((4-aminopiperidin-1-yl)methyl)-N-p-tolylpyrrolo...)Show InChI InChI=1S/C19H24N6/c1-14-2-4-17(5-3-14)23-19-18-15(6-11-25(18)22-13-21-19)12-24-9-7-16(20)8-10-24/h2-6,11,13,16H,7-10,12,20H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333378
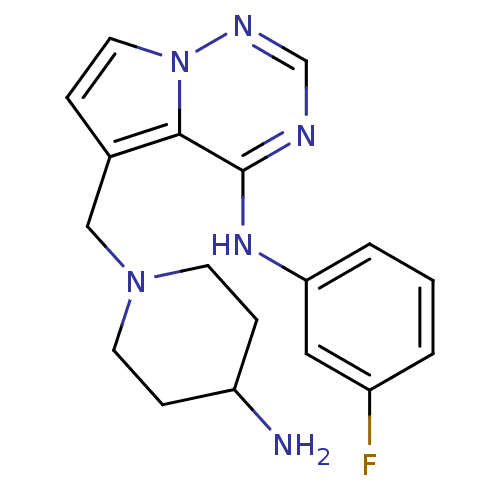
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-fluoropheny...)Show InChI InChI=1S/C18H21FN6/c19-14-2-1-3-16(10-14)23-18-17-13(4-9-25(17)22-12-21-18)11-24-7-5-15(20)6-8-24/h1-4,9-10,12,15H,5-8,11,20H2,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333372
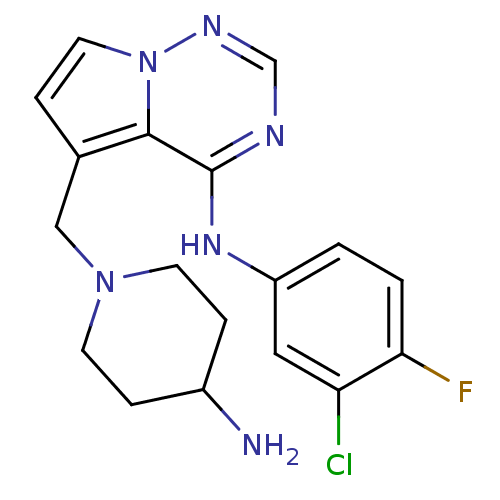
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-chloro-4-fl...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc(F)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C18H20ClFN6/c19-15-9-14(1-2-16(15)20)24-18-17-12(3-8-26(17)23-11-22-18)10-25-6-4-13(21)5-7-25/h1-3,8-9,11,13H,4-7,10,21H2,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333379
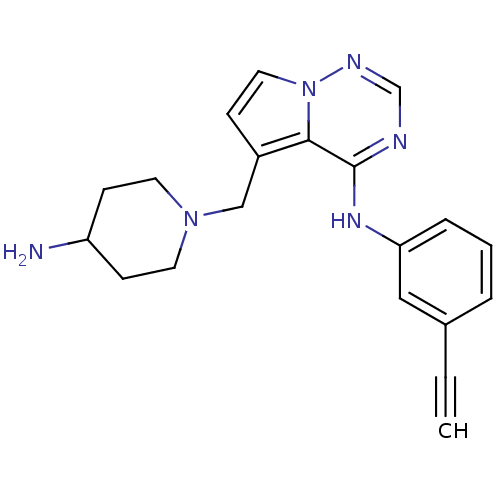
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-ethynylphen...)Show InChI InChI=1S/C20H22N6/c1-2-15-4-3-5-18(12-15)24-20-19-16(6-11-26(19)23-14-22-20)13-25-9-7-17(21)8-10-25/h1,3-6,11-12,14,17H,7-10,13,21H2,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333380
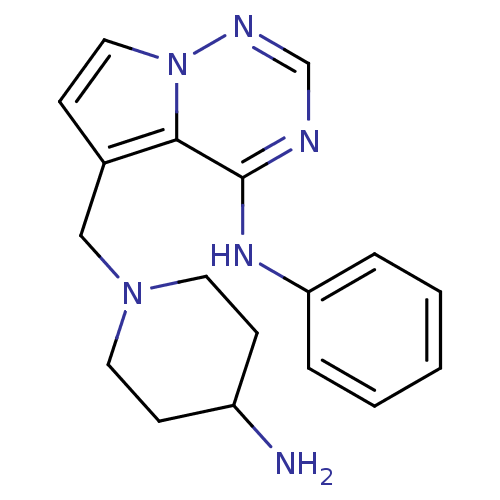
(5-((4-aminopiperidin-1-yl)methyl)-N-phenylpyrrolo[...)Show InChI InChI=1S/C18H22N6/c19-15-7-9-23(10-8-15)12-14-6-11-24-17(14)18(20-13-21-24)22-16-4-2-1-3-5-16/h1-6,11,13,15H,7-10,12,19H2,(H,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50333374

(5-((4-aminopiperidin-1-yl)methyl)-N-(3-chloropheny...)Show InChI InChI=1S/C18H21ClN6/c19-14-2-1-3-16(10-14)23-18-17-13(4-9-25(17)22-12-21-18)11-24-7-5-15(20)6-8-24/h1-4,9-10,12,15H,5-8,11,20H2,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50333372

(5-((4-aminopiperidin-1-yl)methyl)-N-(3-chloro-4-fl...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc(F)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C18H20ClFN6/c19-15-9-14(1-2-16(15)20)24-18-17-12(3-8-26(17)23-11-22-18)10-25-6-4-13(21)5-7-25/h1-3,8-9,11,13H,4-7,10,21H2,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50333379

(5-((4-aminopiperidin-1-yl)methyl)-N-(3-ethynylphen...)Show InChI InChI=1S/C20H22N6/c1-2-15-4-3-5-18(12-15)24-20-19-16(6-11-26(19)23-14-22-20)13-25-9-7-17(21)8-10-25/h1,3-6,11-12,14,17H,7-10,13,21H2,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217195
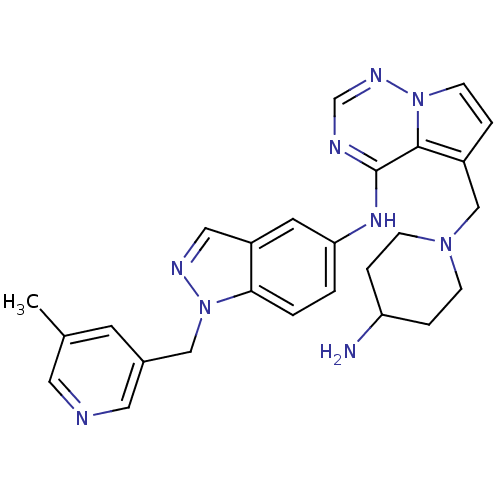
(5-((4-aminopiperidin-1-yl)methyl)-N-(1-((5-methylp...)Show SMILES Cc1cncc(Cn2ncc3cc(Nc4ncnn5ccc(CN6CCC(N)CC6)c45)ccc23)c1 Show InChI InChI=1S/C26H29N9/c1-18-10-19(13-28-12-18)15-35-24-3-2-23(11-21(24)14-30-35)32-26-25-20(4-9-34(25)31-17-29-26)16-33-7-5-22(27)6-8-33/h2-4,9-14,17,22H,5-8,15-16,27H2,1H3,(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50208409
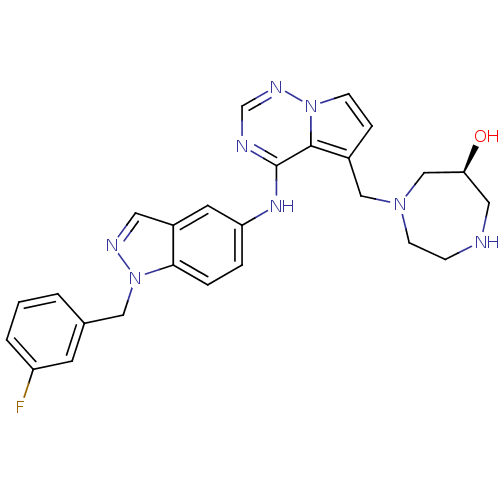
((S)-1-((4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino...)Show SMILES O[C@H]1CNCCN(Cc2ccn3ncnc(Nc4ccc5n(Cc6cccc(F)c6)ncc5c4)c23)C1 Show InChI InChI=1S/C26H27FN8O/c27-21-3-1-2-18(10-21)14-35-24-5-4-22(11-20(24)12-30-35)32-26-25-19(6-8-34(25)31-17-29-26)15-33-9-7-28-13-23(36)16-33/h1-6,8,10-12,17,23,28,36H,7,9,13-16H2,(H,29,31,32)/t23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human HER2 |
Bioorg Med Chem Lett 17: 2828-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.050
BindingDB Entry DOI: 10.7270/Q2TM79SZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299488
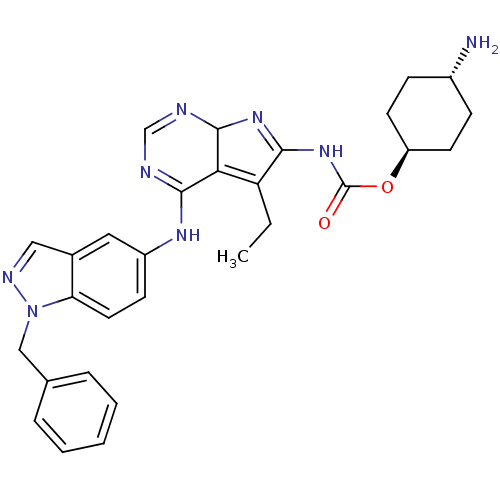
(CHEMBL565467 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)O[C@H]1CC[C@H](N)CC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |r,wU:11.11,wD:14.15,c:5,20,22,t:2,(-3.88,-32.47,;-4.85,-33.66,;-4.3,-35.1,;-2.81,-35.51,;-2.73,-37.06,;-4.18,-37.6,;-5.15,-36.4,;-6.69,-36.47,;-7.4,-37.84,;-6.56,-39.14,;-8.93,-37.92,;-9.64,-39.29,;-8.8,-40.58,;-9.51,-41.95,;-11.05,-42.02,;-11.77,-43.39,;-11.89,-40.72,;-11.18,-39.36,;-1.37,-37.75,;-.08,-36.93,;-.15,-35.39,;-1.52,-34.68,;-1.59,-33.14,;-.29,-32.31,;-.37,-30.78,;.92,-29.94,;2.3,-30.65,;3.74,-30.11,;4.14,-28.62,;5.64,-28.24,;6.72,-29.34,;8.21,-28.95,;8.62,-27.47,;7.53,-26.37,;6.04,-26.76,;4.7,-31.31,;3.85,-32.59,;2.37,-32.19,;1.07,-33.02,)| Show InChI InChI=1S/C29H32N8O2/c1-2-23-25-27(31-17-32-28(25)35-26(23)36-29(38)39-22-11-8-20(30)9-12-22)34-21-10-13-24-19(14-21)15-33-37(24)16-18-6-4-3-5-7-18/h3-7,10,13-15,17,20,22,28H,2,8-9,11-12,16,30H2,1H3,(H,31,32,34)(H,35,36,38)/t20-,22-,28? | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299487
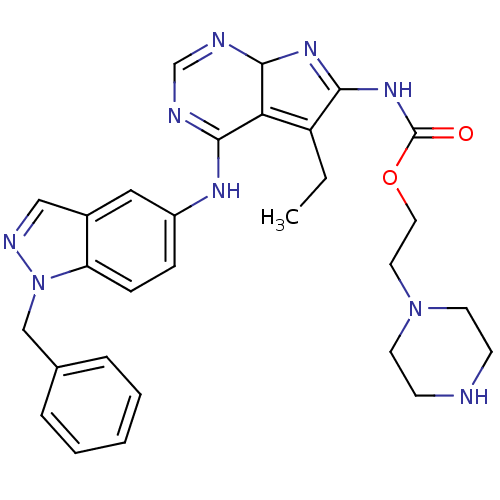
(CHEMBL565714 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCCN1CCNCC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,21,23,t:2| Show InChI InChI=1S/C29H33N9O2/c1-2-23-25-27(34-22-8-9-24-21(16-22)17-33-38(24)18-20-6-4-3-5-7-20)31-19-32-28(25)35-26(23)36-29(39)40-15-14-37-12-10-30-11-13-37/h3-9,16-17,19,28,30H,2,10-15,18H2,1H3,(H,31,32,34)(H,35,36,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217188
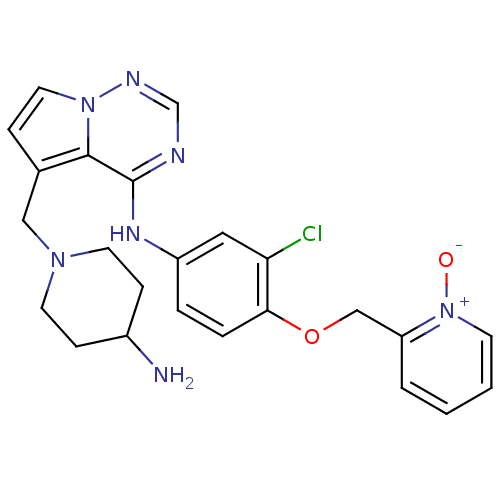
(CHEMBL248322 | [5-(4-amino-piperidin-1-ylmethyl)-p...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc(OCc5cccc[n+]5[O-])c(Cl)c4)c23)CC1 Show InChI InChI=1S/C24H26ClN7O2/c25-21-13-19(4-5-22(21)34-15-20-3-1-2-9-32(20)33)29-24-23-17(6-12-31(23)28-16-27-24)14-30-10-7-18(26)8-11-30/h1-6,9,12-13,16,18H,7-8,10-11,14-15,26H2,(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50204120
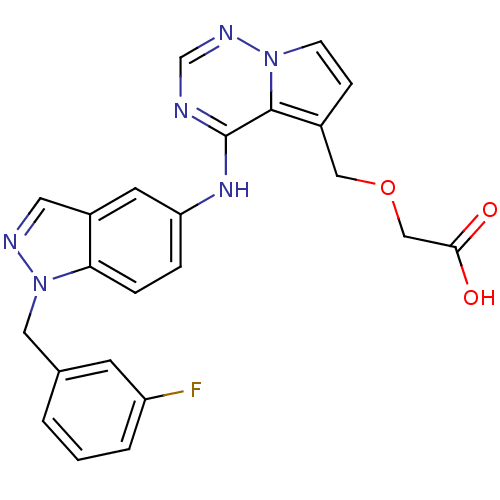
(2-((4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino)pyr...)Show SMILES OC(=O)COCc1ccn2ncnc(Nc3ccc4n(Cc5cccc(F)c5)ncc4c3)c12 Show InChI InChI=1S/C23H19FN6O3/c24-18-3-1-2-15(8-18)11-30-20-5-4-19(9-17(20)10-26-30)28-23-22-16(12-33-13-21(31)32)6-7-29(22)27-14-25-23/h1-10,14H,11-13H2,(H,31,32)(H,25,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cytoplasmic HER2 kinase expressed in Sf9 cells |
Bioorg Med Chem Lett 17: 2036-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.002
BindingDB Entry DOI: 10.7270/Q2GT5MVS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50299489
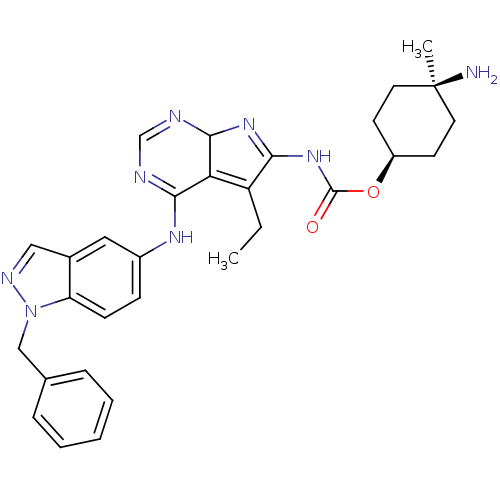
(CHEMBL567197 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)O[C@H]1CC[C@@](C)(N)CC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |r,wU:11.11,14.16,wD:14.15,c:5,21,23,t:2,(15.77,-33.64,;14.8,-34.84,;15.34,-36.28,;16.84,-36.68,;16.91,-38.23,;15.46,-38.78,;14.49,-37.57,;12.96,-37.65,;12.25,-39.02,;13.09,-40.31,;10.71,-39.09,;10.01,-40.46,;10.84,-41.75,;10.13,-43.12,;8.59,-43.19,;7.05,-43.18,;9.35,-44.52,;7.76,-41.89,;8.47,-40.53,;18.28,-38.93,;19.57,-38.1,;19.49,-36.56,;18.13,-35.85,;18.06,-34.31,;19.35,-33.48,;19.28,-31.95,;20.57,-31.12,;21.94,-31.82,;23.38,-31.28,;23.79,-29.8,;25.28,-29.41,;26.36,-30.51,;27.85,-30.13,;28.26,-28.64,;27.17,-27.54,;25.69,-27.93,;24.34,-32.49,;23.5,-33.77,;22.01,-33.36,;20.72,-34.19,)| Show InChI InChI=1S/C30H34N8O2/c1-3-23-25-27(35-21-9-10-24-20(15-21)16-34-38(24)17-19-7-5-4-6-8-19)32-18-33-28(25)36-26(23)37-29(39)40-22-11-13-30(2,31)14-12-22/h4-10,15-16,18,22,28H,3,11-14,17,31H2,1-2H3,(H,32,33,35)(H,36,37,39)/t22-,28?,30+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER1 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50217195

(5-((4-aminopiperidin-1-yl)methyl)-N-(1-((5-methylp...)Show SMILES Cc1cncc(Cn2ncc3cc(Nc4ncnn5ccc(CN6CCC(N)CC6)c45)ccc23)c1 Show InChI InChI=1S/C26H29N9/c1-18-10-19(13-28-12-18)15-35-24-3-2-23(11-21(24)14-30-35)32-26-25-20(4-9-34(25)31-17-29-26)16-33-7-5-22(27)6-8-33/h2-4,9-14,17,22H,5-8,15-16,27H2,1H3,(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant EGFR expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333381
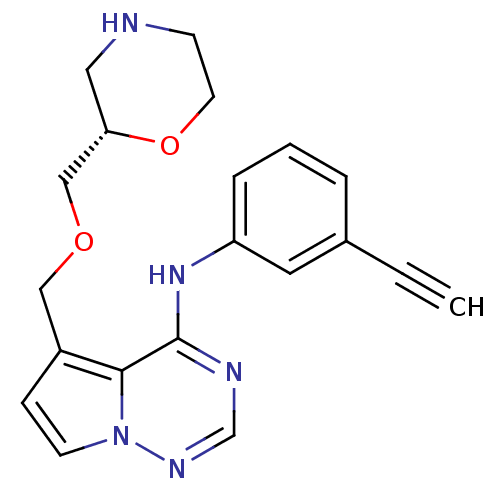
((S)-N-(3-ethynylphenyl)-5-((morpholin-2-ylmethoxy)...)Show SMILES C#Cc1cccc(Nc2ncnn3ccc(COC[C@@H]4CNCCO4)c23)c1 |r| Show InChI InChI=1S/C20H21N5O2/c1-2-15-4-3-5-17(10-15)24-20-19-16(6-8-25(19)23-14-22-20)12-26-13-18-11-21-7-9-27-18/h1,3-6,8,10,14,18,21H,7,9,11-13H2,(H,22,23,24)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333382

(5-((4-aminopiperidin-1-yl)methyl)-N-o-tolylpyrrolo...)Show InChI InChI=1S/C19H24N6/c1-14-4-2-3-5-17(14)23-19-18-15(6-11-25(18)22-13-21-19)12-24-9-7-16(20)8-10-24/h2-6,11,13,16H,7-10,12,20H2,1H3,(H,21,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50333376

(5-((4-aminopiperidin-1-yl)methyl)-N-m-tolylpyrrolo...)Show InChI InChI=1S/C19H24N6/c1-14-3-2-4-17(11-14)23-19-18-15(5-10-25(18)22-13-21-19)12-24-8-6-16(20)7-9-24/h2-5,10-11,13,16H,6-9,12,20H2,1H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299489

(CHEMBL567197 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)O[C@H]1CC[C@@](C)(N)CC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |r,wU:11.11,14.16,wD:14.15,c:5,21,23,t:2,(15.77,-33.64,;14.8,-34.84,;15.34,-36.28,;16.84,-36.68,;16.91,-38.23,;15.46,-38.78,;14.49,-37.57,;12.96,-37.65,;12.25,-39.02,;13.09,-40.31,;10.71,-39.09,;10.01,-40.46,;10.84,-41.75,;10.13,-43.12,;8.59,-43.19,;7.05,-43.18,;9.35,-44.52,;7.76,-41.89,;8.47,-40.53,;18.28,-38.93,;19.57,-38.1,;19.49,-36.56,;18.13,-35.85,;18.06,-34.31,;19.35,-33.48,;19.28,-31.95,;20.57,-31.12,;21.94,-31.82,;23.38,-31.28,;23.79,-29.8,;25.28,-29.41,;26.36,-30.51,;27.85,-30.13,;28.26,-28.64,;27.17,-27.54,;25.69,-27.93,;24.34,-32.49,;23.5,-33.77,;22.01,-33.36,;20.72,-34.19,)| Show InChI InChI=1S/C30H34N8O2/c1-3-23-25-27(35-21-9-10-24-20(15-21)16-34-38(24)17-19-7-5-4-6-8-19)32-18-33-28(25)36-26(23)37-29(39)40-22-11-13-30(2,31)14-12-22/h4-10,15-16,18,22,28H,3,11-14,17,31H2,1-2H3,(H,32,33,35)(H,36,37,39)/t22-,28?,30+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM13911
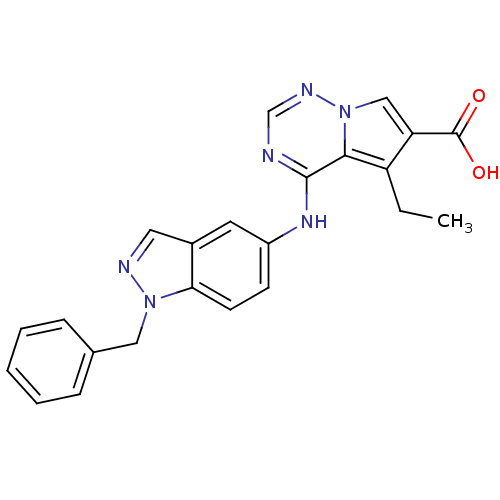
(4-[(1-benzyl-1H-indazol-5-yl)amino]-5-ethylpyrrolo...)Show SMILES CCc1c(cn2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c12)C(O)=O Show InChI InChI=1S/C23H20N6O2/c1-2-18-19(23(30)31)13-29-21(18)22(24-14-26-29)27-17-8-9-20-16(10-17)11-25-28(20)12-15-6-4-3-5-7-15/h3-11,13-14H,2,12H2,1H3,(H,30,31)(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against EGFR cytoplasmic sequence expressed in Sf9 cells |
Bioorg Med Chem Lett 15: 4774-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.027
BindingDB Entry DOI: 10.7270/Q2XD1172 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM13911

(4-[(1-benzyl-1H-indazol-5-yl)amino]-5-ethylpyrrolo...)Show SMILES CCc1c(cn2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c12)C(O)=O Show InChI InChI=1S/C23H20N6O2/c1-2-18-19(23(30)31)13-29-21(18)22(24-14-26-29)27-17-8-9-20-16(10-17)11-25-28(20)12-15-6-4-3-5-7-15/h3-11,13-14H,2,12H2,1H3,(H,30,31)(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
The kinase activity was determined by using recombinant enzyme incubating with its substrate in the presence of test compound and ATP/[gamma-33P] ATP... |
Bioorg Med Chem Lett 15: 4774-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.027
BindingDB Entry DOI: 10.7270/Q2XD1172 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299490

(CHEMBL567873 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OC[C@@H]1COCCN1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |r,c:5,20,22,t:2| Show InChI InChI=1S/C28H30N8O3/c1-2-22-24-26(30-17-31-27(24)34-25(22)35-28(37)39-16-21-15-38-11-10-29-21)33-20-8-9-23-19(12-20)13-32-36(23)14-18-6-4-3-5-7-18/h3-9,12-13,17,21,27,29H,2,10-11,14-16H2,1H3,(H,30,31,33)(H,34,35,37)/t21-,27?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50299487

(CHEMBL565714 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCCN1CCNCC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,21,23,t:2| Show InChI InChI=1S/C29H33N9O2/c1-2-23-25-27(34-22-8-9-24-21(16-22)17-33-38(24)18-20-6-4-3-5-7-20)31-19-32-28(25)35-26(23)36-29(39)40-15-14-37-12-10-30-11-13-37/h3-9,16-17,19,28,30H,2,10-15,18H2,1H3,(H,31,32,34)(H,35,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER1 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217206
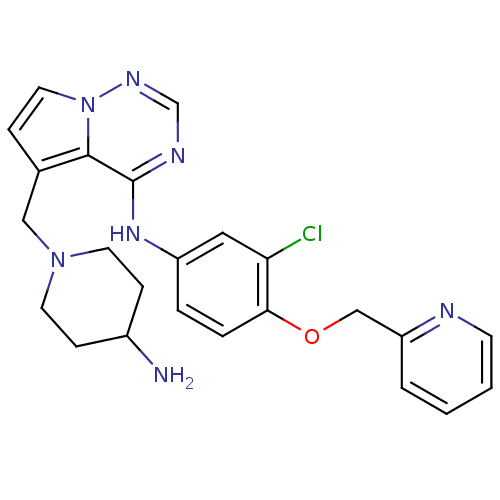
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-chloro-4-(p...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C24H26ClN7O/c25-21-13-19(4-5-22(21)33-15-20-3-1-2-9-27-20)30-24-23-17(6-12-32(23)29-16-28-24)14-31-10-7-18(26)8-11-31/h1-6,9,12-13,16,18H,7-8,10-11,14-15,26H2,(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299491
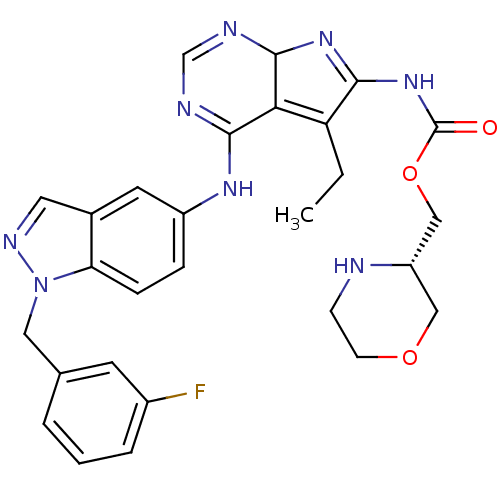
(CHEMBL583403 | [4-[[1-(3-fluorophenyl)methyl]-1H-i...)Show SMILES CCC1=C2C(N=C1NC(=O)OC[C@@H]1COCCN1)N=CN=C2Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 |r,c:5,20,22,t:2| Show InChI InChI=1S/C28H29FN8O3/c1-2-22-24-26(31-16-32-27(24)35-25(22)36-28(38)40-15-21-14-39-9-8-30-21)34-20-6-7-23-18(11-20)12-33-37(23)13-17-4-3-5-19(29)10-17/h3-7,10-12,16,21,27,30H,2,8-9,13-15H2,1H3,(H,31,32,34)(H,35,36,38)/t21-,27?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333373
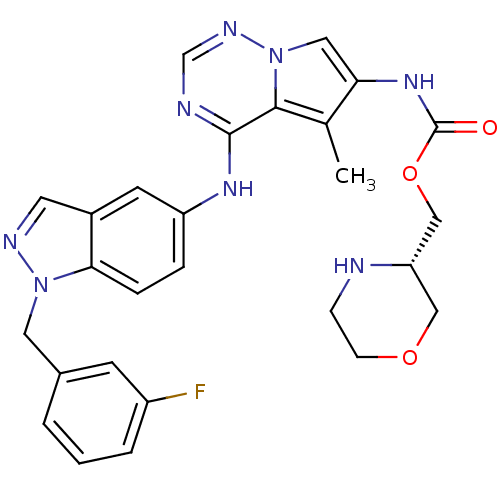
(CHEMBL1645462 | [4-[[1-(3-fluorophenyl)methyl]-1H-...)Show SMILES Cc1c(NC(=O)OC[C@@H]2COCCN2)cn2ncnc(Nc3ccc4n(Cc5cccc(F)c5)ncc4c3)c12 |r| Show InChI InChI=1S/C27H27FN8O3/c1-17-23(34-27(37)39-15-22-14-38-8-7-29-22)13-36-25(17)26(30-16-32-36)33-21-5-6-24-19(10-21)11-31-35(24)12-18-3-2-4-20(28)9-18/h2-6,9-11,13,16,22,29H,7-8,12,14-15H2,1H3,(H,34,37)(H,30,32,33)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217191
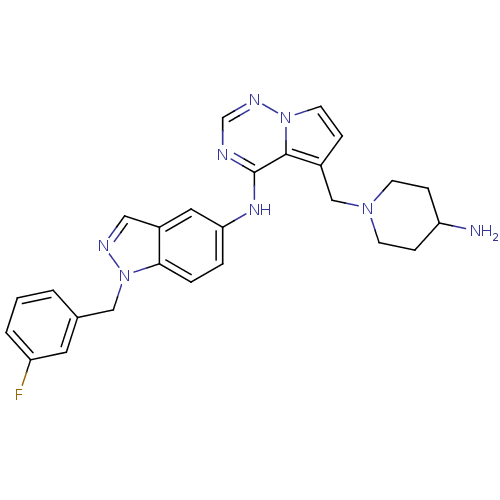
(CHEMBL248108 | N-(1-(3-fluorobenzyl)-1H-indazol-5-...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc5n(Cc6cccc(F)c6)ncc5c4)c23)CC1 Show InChI InChI=1S/C26H27FN8/c27-21-3-1-2-18(12-21)15-35-24-5-4-23(13-20(24)14-30-35)32-26-25-19(6-11-34(25)31-17-29-26)16-33-9-7-22(28)8-10-33/h1-6,11-14,17,22H,7-10,15-16,28H2,(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50299491

(CHEMBL583403 | [4-[[1-(3-fluorophenyl)methyl]-1H-i...)Show SMILES CCC1=C2C(N=C1NC(=O)OC[C@@H]1COCCN1)N=CN=C2Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 |r,c:5,20,22,t:2| Show InChI InChI=1S/C28H29FN8O3/c1-2-22-24-26(31-16-32-27(24)35-25(22)36-28(38)40-15-21-14-39-9-8-30-21)34-20-6-7-23-18(11-20)12-33-37(23)13-17-4-3-5-19(29)10-17/h3-7,10-12,16,21,27,30H,2,8-9,13-15H2,1H3,(H,31,32,34)(H,35,36,38)/t21-,27?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER1 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217191

(CHEMBL248108 | N-(1-(3-fluorobenzyl)-1H-indazol-5-...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc5n(Cc6cccc(F)c6)ncc5c4)c23)CC1 Show InChI InChI=1S/C26H27FN8/c27-21-3-1-2-18(12-21)15-35-24-5-4-23(13-20(24)14-30-35)32-26-25-19(6-11-34(25)31-17-29-26)16-33-9-7-22(28)8-10-33/h1-6,11-14,17,22H,7-10,15-16,28H2,(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299492
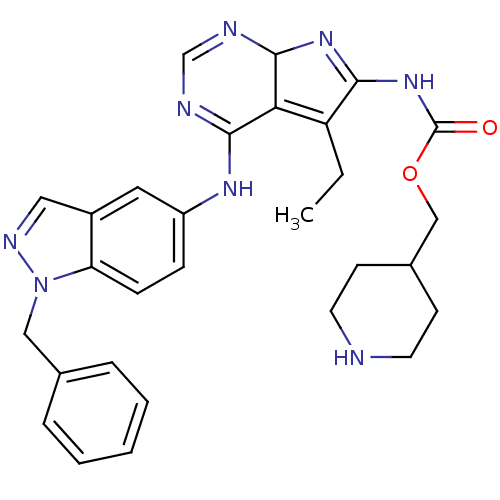
(CHEMBL583218 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCC1CCNCC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,20,22,t:2| Show InChI InChI=1S/C29H32N8O2/c1-2-23-25-27(31-18-32-28(25)35-26(23)36-29(38)39-17-20-10-12-30-13-11-20)34-22-8-9-24-21(14-22)15-33-37(24)16-19-6-4-3-5-7-19/h3-9,14-15,18,20,28,30H,2,10-13,16-17H2,1H3,(H,31,32,34)(H,35,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50299488

(CHEMBL565467 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)O[C@H]1CC[C@H](N)CC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |r,wU:11.11,wD:14.15,c:5,20,22,t:2,(-3.88,-32.47,;-4.85,-33.66,;-4.3,-35.1,;-2.81,-35.51,;-2.73,-37.06,;-4.18,-37.6,;-5.15,-36.4,;-6.69,-36.47,;-7.4,-37.84,;-6.56,-39.14,;-8.93,-37.92,;-9.64,-39.29,;-8.8,-40.58,;-9.51,-41.95,;-11.05,-42.02,;-11.77,-43.39,;-11.89,-40.72,;-11.18,-39.36,;-1.37,-37.75,;-.08,-36.93,;-.15,-35.39,;-1.52,-34.68,;-1.59,-33.14,;-.29,-32.31,;-.37,-30.78,;.92,-29.94,;2.3,-30.65,;3.74,-30.11,;4.14,-28.62,;5.64,-28.24,;6.72,-29.34,;8.21,-28.95,;8.62,-27.47,;7.53,-26.37,;6.04,-26.76,;4.7,-31.31,;3.85,-32.59,;2.37,-32.19,;1.07,-33.02,)| Show InChI InChI=1S/C29H32N8O2/c1-2-23-25-27(31-17-32-28(25)35-26(23)36-29(38)39-22-11-8-20(30)9-12-22)34-21-10-13-24-19(14-21)15-33-37(24)16-18-6-4-3-5-7-18/h3-7,10,13-15,17,20,22,28H,2,8-9,11-12,16,30H2,1H3,(H,31,32,34)(H,35,36,38)/t20-,22-,28? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER1 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50333373

(CHEMBL1645462 | [4-[[1-(3-fluorophenyl)methyl]-1H-...)Show SMILES Cc1c(NC(=O)OC[C@@H]2COCCN2)cn2ncnc(Nc3ccc4n(Cc5cccc(F)c5)ncc4c3)c12 |r| Show InChI InChI=1S/C27H27FN8O3/c1-17-23(34-27(37)39-15-22-14-38-8-7-29-22)13-36-25(17)26(30-16-32-36)33-21-5-6-24-19(10-21)11-31-35(24)12-18-3-2-4-20(28)9-18/h2-6,9-11,13,16,22,29H,7-8,12,14-15H2,1H3,(H,34,37)(H,30,32,33)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HER2 |
Bioorg Med Chem Lett 21: 781-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.100
BindingDB Entry DOI: 10.7270/Q2X34XQ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50208409

((S)-1-((4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino...)Show SMILES O[C@H]1CNCCN(Cc2ccn3ncnc(Nc4ccc5n(Cc6cccc(F)c6)ncc5c4)c23)C1 Show InChI InChI=1S/C26H27FN8O/c27-21-3-1-2-18(10-21)14-35-24-5-4-22(11-20(24)12-30-35)32-26-25-19(6-8-34(25)31-17-29-26)15-33-9-7-28-13-23(36)16-33/h1-6,8,10-12,17,23,28,36H,7,9,13-16H2,(H,29,31,32)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR |
Bioorg Med Chem Lett 17: 2828-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.050
BindingDB Entry DOI: 10.7270/Q2TM79SZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217186
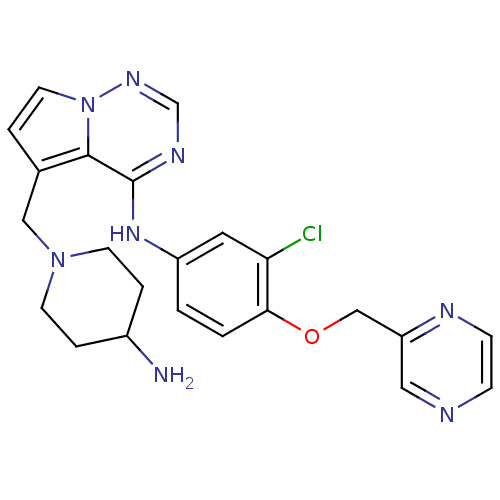
(5-((4-aminopiperidin-1-yl)methyl)-N-(3-chloro-4-(p...)Show SMILES NC1CCN(Cc2ccn3ncnc(Nc4ccc(OCc5cnccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C23H25ClN8O/c24-20-11-18(1-2-21(20)33-14-19-12-26-6-7-27-19)30-23-22-16(3-10-32(22)29-15-28-23)13-31-8-4-17(25)5-9-31/h1-3,6-7,10-12,15,17H,4-5,8-9,13-14,25H2,(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50208401
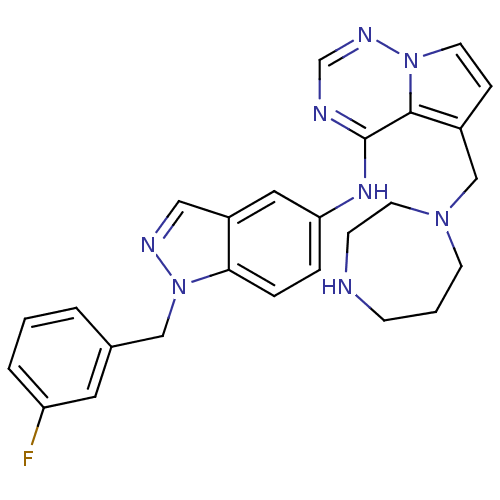
(5-((1,4-diazepan-1-yl)methyl)-N-(1-(3-fluorobenzyl...)Show SMILES Fc1cccc(Cn2ncc3cc(Nc4ncnn5ccc(CN6CCCNCC6)c45)ccc23)c1 Show InChI InChI=1S/C26H27FN8/c27-22-4-1-3-19(13-22)16-35-24-6-5-23(14-21(24)15-30-35)32-26-25-20(7-11-34(25)31-18-29-26)17-33-10-2-8-28-9-12-33/h1,3-7,11,13-15,18,28H,2,8-10,12,16-17H2,(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50208401

(5-((1,4-diazepan-1-yl)methyl)-N-(1-(3-fluorobenzyl...)Show SMILES Fc1cccc(Cn2ncc3cc(Nc4ncnn5ccc(CN6CCCNCC6)c45)ccc23)c1 Show InChI InChI=1S/C26H27FN8/c27-22-4-1-3-19(13-22)16-35-24-6-5-23(14-21(24)15-30-35)32-26-25-20(7-11-34(25)31-18-29-26)17-33-10-2-8-28-9-12-33/h1,3-7,11,13-15,18,28H,2,8-10,12,16-17H2,(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human HER2 |
Bioorg Med Chem Lett 17: 2828-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.050
BindingDB Entry DOI: 10.7270/Q2TM79SZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299493
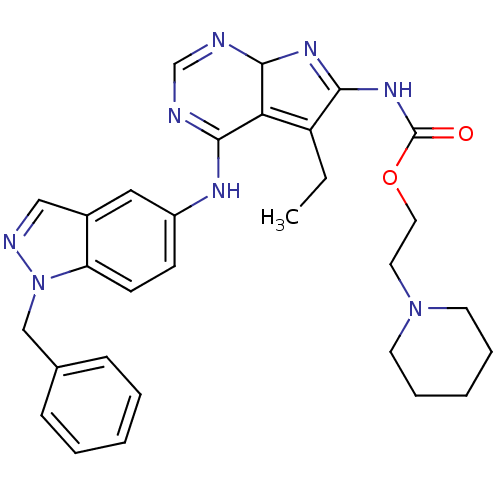
(CHEMBL566350 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCCN1CCCCC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,21,23,t:2| Show InChI InChI=1S/C30H34N8O2/c1-2-24-26-28(34-23-11-12-25-22(17-23)18-33-38(25)19-21-9-5-3-6-10-21)31-20-32-29(26)35-27(24)36-30(39)40-16-15-37-13-7-4-8-14-37/h3,5-6,9-12,17-18,20,29H,2,4,7-8,13-16,19H2,1H3,(H,31,32,34)(H,35,36,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299494
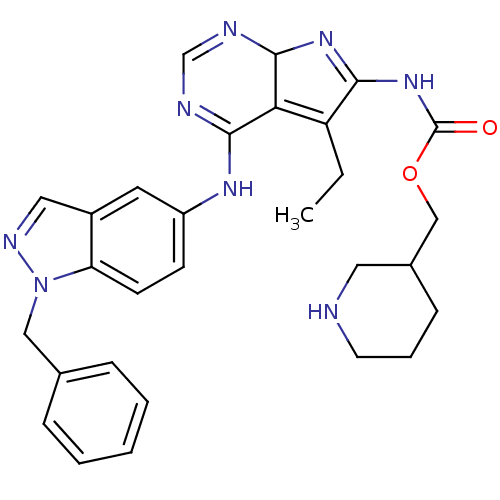
(CHEMBL584714 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCC1CCCNC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,20,22,t:2| Show InChI InChI=1S/C29H32N8O2/c1-2-23-25-27(31-18-32-28(25)35-26(23)36-29(38)39-17-20-9-6-12-30-14-20)34-22-10-11-24-21(13-22)15-33-37(24)16-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,15,18,20,28,30H,2,6,9,12,14,16-17H2,1H3,(H,31,32,34)(H,35,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50299492

(CHEMBL583218 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCC1CCNCC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,20,22,t:2| Show InChI InChI=1S/C29H32N8O2/c1-2-23-25-27(31-18-32-28(25)35-26(23)36-29(38)39-17-20-10-12-30-13-11-20)34-22-8-9-24-21(14-22)15-33-37(24)16-19-6-4-3-5-7-19/h3-9,14-15,18,20,28,30H,2,10-13,16-17H2,1H3,(H,31,32,34)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER1 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50299495
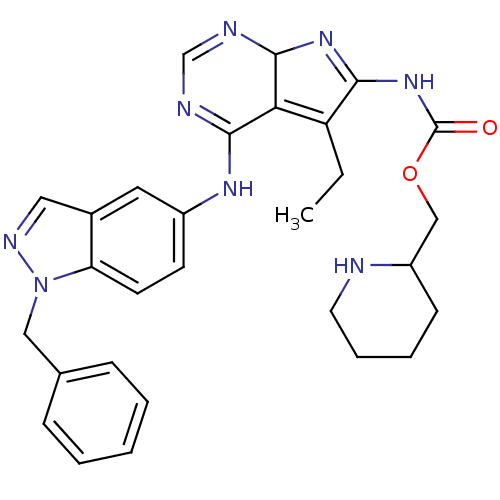
(CHEMBL566559 | {4-(1-Benzyl-1H-indazol-5-ylamino)-...)Show SMILES CCC1=C2C(N=C1NC(=O)OCC1CCCCN1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1 |c:5,20,22,t:2| Show InChI InChI=1S/C29H32N8O2/c1-2-23-25-27(31-18-32-28(25)35-26(23)36-29(38)39-17-22-10-6-7-13-30-22)34-21-11-12-24-20(14-21)15-33-37(24)16-19-8-4-3-5-9-19/h3-5,8-9,11-12,14-15,18,22,28,30H,2,6-7,10,13,16-17H2,1H3,(H,31,32,34)(H,35,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 6527-30 (2009)
Article DOI: 10.1021/jm9010065
BindingDB Entry DOI: 10.7270/Q2542PH9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50235548

(CHEMBL254280 | N1-(4-(6-(3-(dimethylamino)propoxy)...)Show SMILES CN(C)CCCOc1cn2ncnc(Oc3ccc(NC(=O)CC(=O)Nc4ccc(F)cc4)cc3F)c2c1C Show InChI InChI=1S/C27H28F2N6O4/c1-17-23(38-12-4-11-34(2)3)15-35-26(17)27(30-16-31-35)39-22-10-9-20(13-21(22)29)33-25(37)14-24(36)32-19-7-5-18(28)6-8-19/h5-10,13,15-16H,4,11-12,14H2,1-3H3,(H,32,36)(H,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Met expressed in insect cell-baculovirus expression system |
Bioorg Med Chem Lett 18: 1945-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.121
BindingDB Entry DOI: 10.7270/Q2125SFV |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50235540
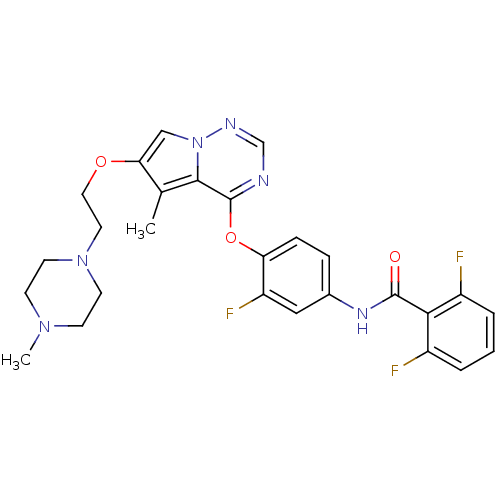
(2,6-difluoro-N-(3-fluoro-4-(5-methyl-6-(2-(4-methy...)Show SMILES CN1CCN(CCOc2cn3ncnc(Oc4ccc(NC(=O)c5c(F)cccc5F)cc4F)c3c2C)CC1 Show InChI InChI=1S/C27H27F3N6O3/c1-17-23(38-13-12-35-10-8-34(2)9-11-35)15-36-25(17)27(31-16-32-36)39-22-7-6-18(14-21(22)30)33-26(37)24-19(28)4-3-5-20(24)29/h3-7,14-16H,8-13H2,1-2H3,(H,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Met expressed in insect cell-baculovirus expression system |
Bioorg Med Chem Lett 18: 1945-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.121
BindingDB Entry DOI: 10.7270/Q2125SFV |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50235572
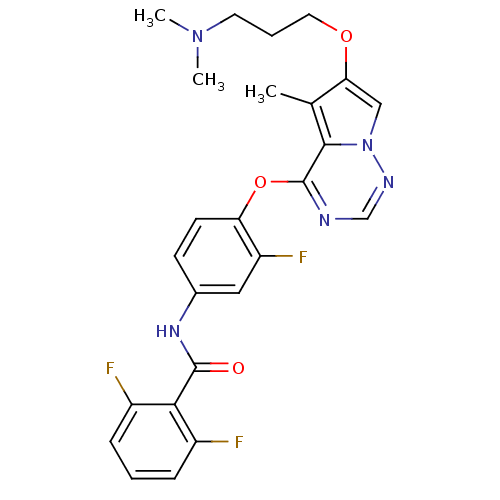
(CHEMBL400332 | N-(4-(6-(3-(dimethylamino)propoxy)-...)Show SMILES CN(C)CCCOc1cn2ncnc(Oc3ccc(NC(=O)c4c(F)cccc4F)cc3F)c2c1C Show InChI InChI=1S/C25H24F3N5O3/c1-15-21(35-11-5-10-32(2)3)13-33-23(15)25(29-14-30-33)36-20-9-8-16(12-19(20)28)31-24(34)22-17(26)6-4-7-18(22)27/h4,6-9,12-14H,5,10-11H2,1-3H3,(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Met expressed in insect cell-baculovirus expression system |
Bioorg Med Chem Lett 18: 1945-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.121
BindingDB Entry DOI: 10.7270/Q2125SFV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM13921
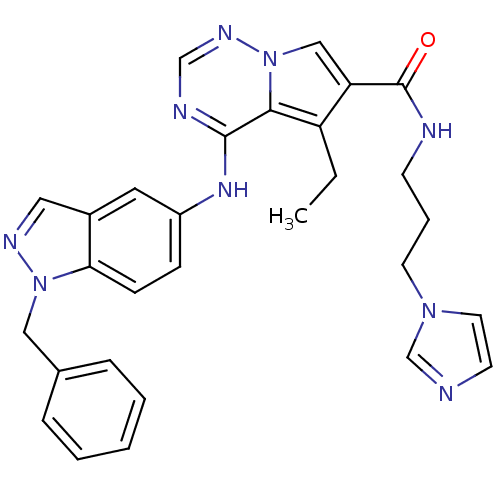
(4-[(1-benzyl-1H-indazol-5-yl)amino]-5-ethyl-N-[3-(...)Show SMILES CCc1c(cn2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c12)C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C29H29N9O/c1-2-24-25(29(39)31-11-6-13-36-14-12-30-20-36)18-38-27(24)28(32-19-34-38)35-23-9-10-26-22(15-23)16-33-37(26)17-21-7-4-3-5-8-21/h3-5,7-10,12,14-16,18-20H,2,6,11,13,17H2,1H3,(H,31,39)(H,32,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
The kinase activity was determined by using recombinant enzyme incubating with its substrate in the presence of test compound and ATP/[gamma-33P] ATP... |
Bioorg Med Chem Lett 15: 4774-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.027
BindingDB Entry DOI: 10.7270/Q2XD1172 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data