

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526620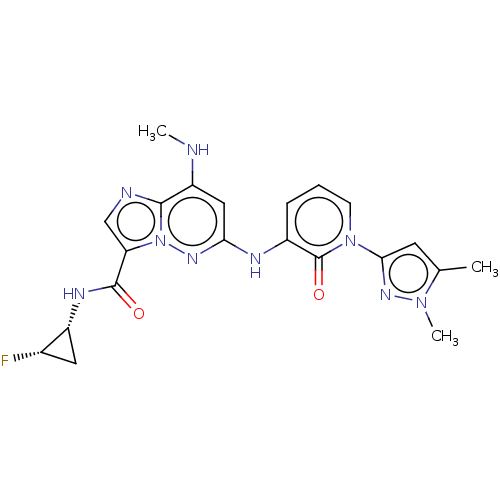 (CHEMBL4460368) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526622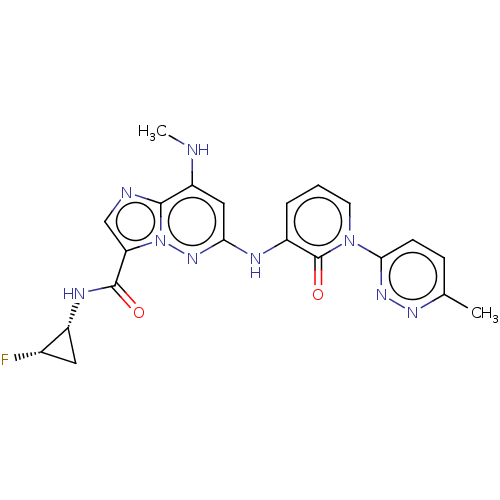 (CHEMBL4442827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526613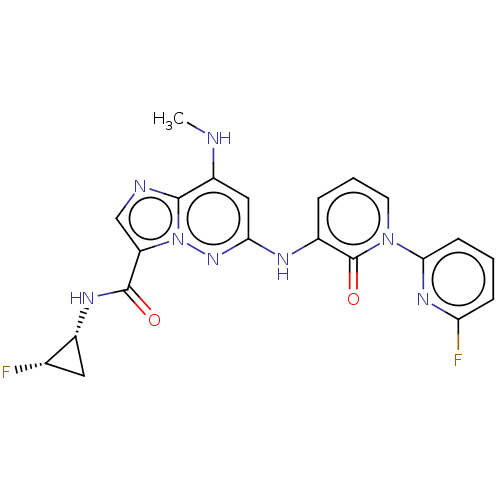 (CHEMBL4571920) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526619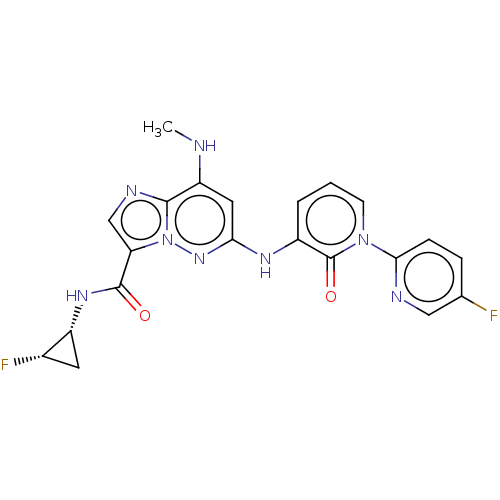 (CHEMBL4439957) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526615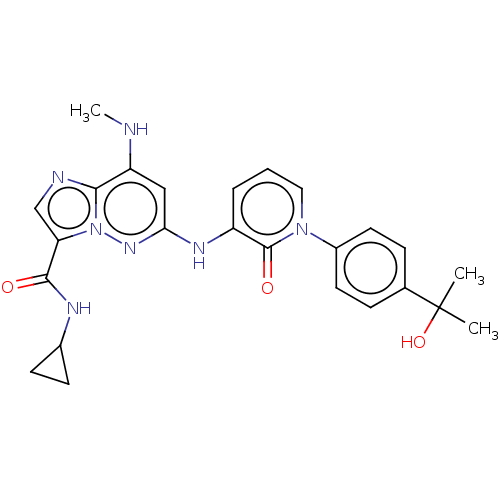 (CHEMBL4434711) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582801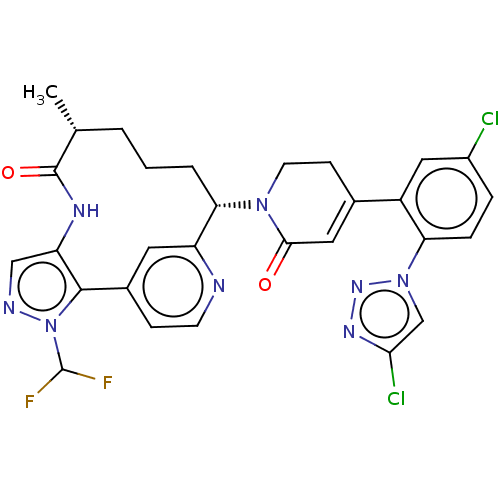 (CHEMBL5076656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526606 (CHEMBL4532948 | US11174264, Compound I-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526624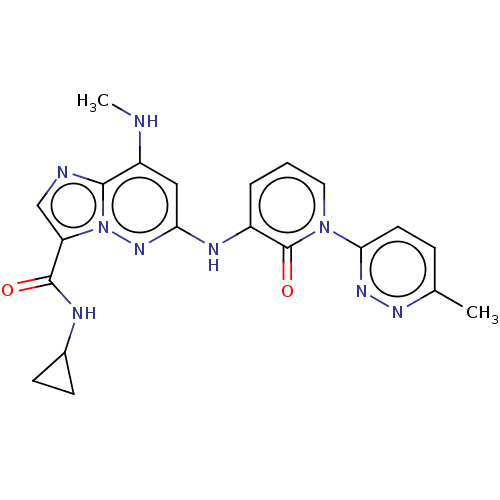 (CHEMBL4443010) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247411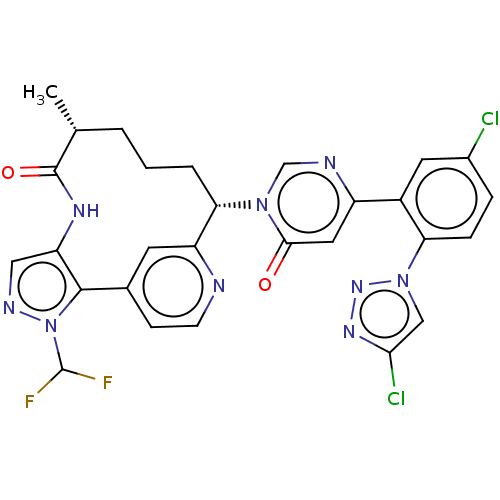 (US10336754, Example 353 | US11053247, Example 353 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526617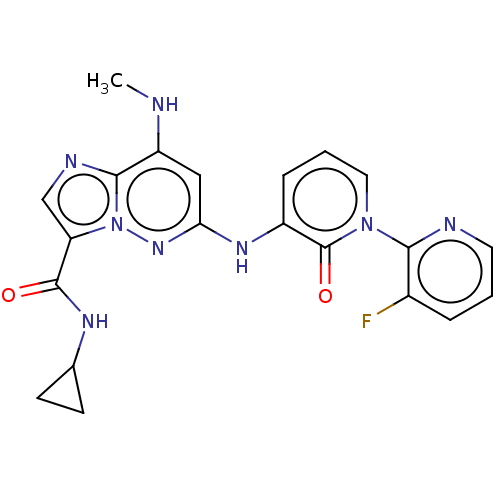 (CHEMBL4435047) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582799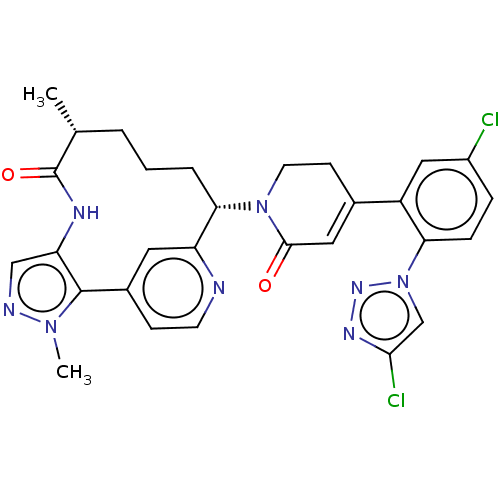 (CHEMBL5094166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526603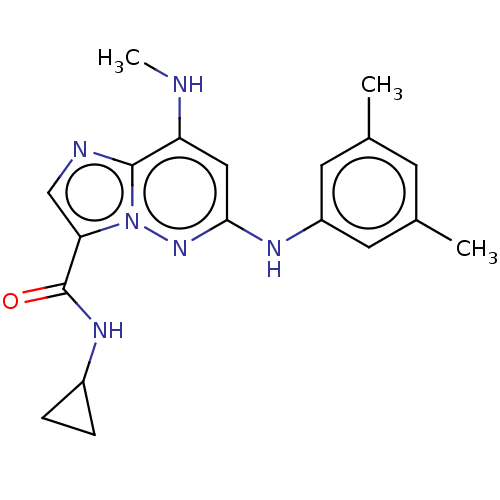 (CHEMBL4293907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526621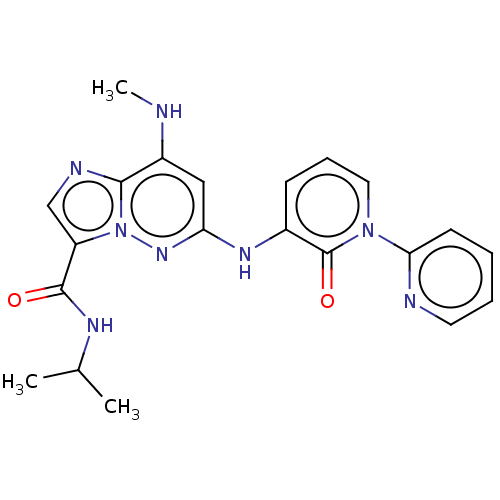 (CHEMBL4438107) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526607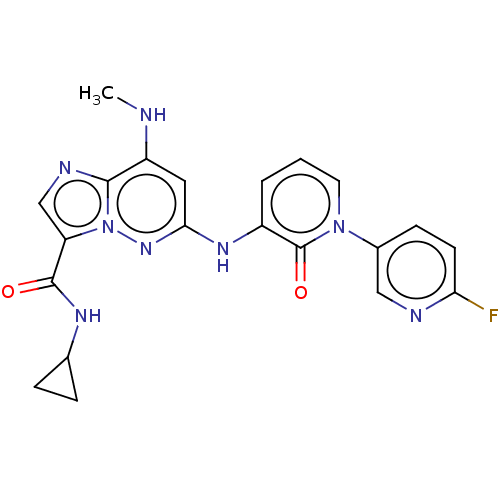 (CHEMBL4474801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526604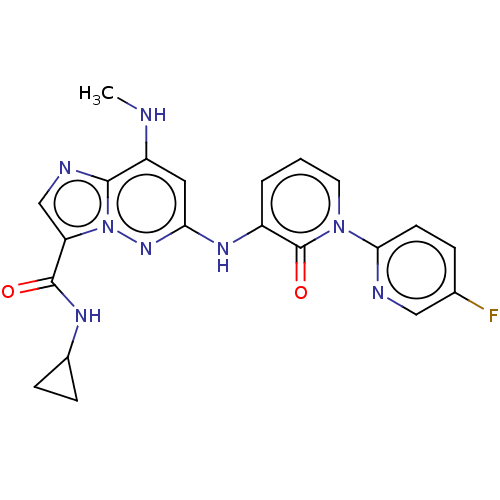 (CHEMBL4561123) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582800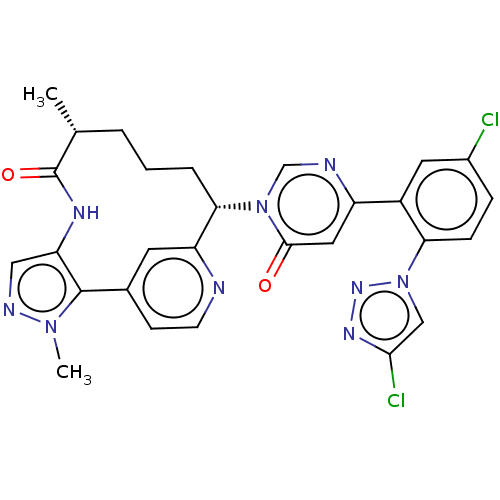 (CHEMBL5093567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526610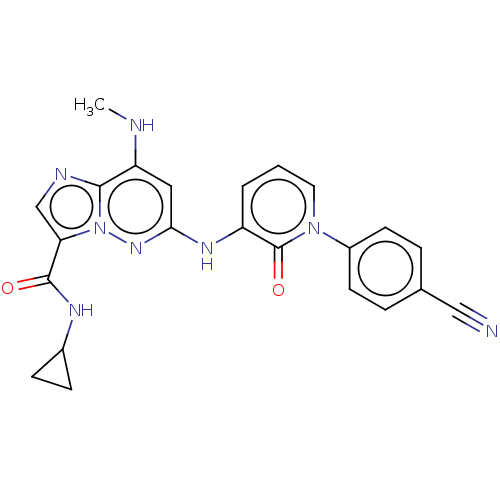 (CHEMBL4453441) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526616 (CHEMBL4579439) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586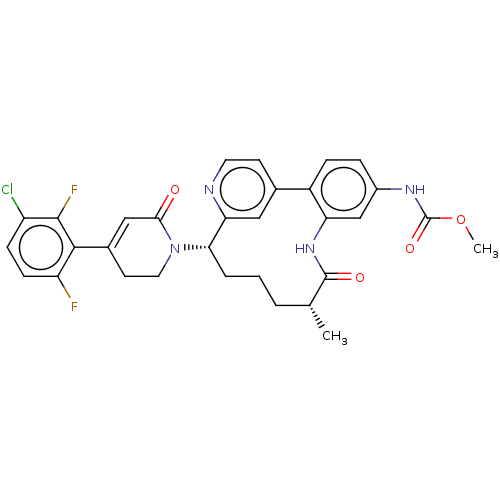 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526605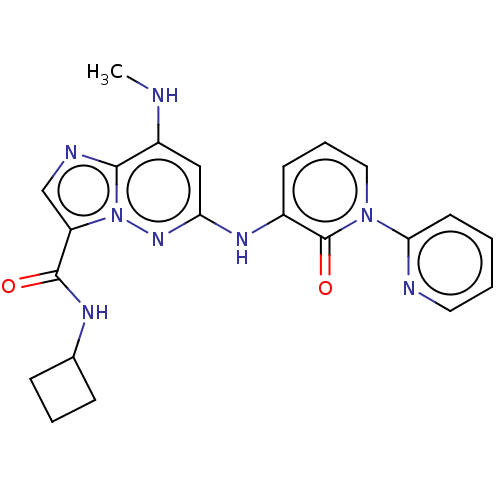 (CHEMBL4438296) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526612 (CHEMBL4543066) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526611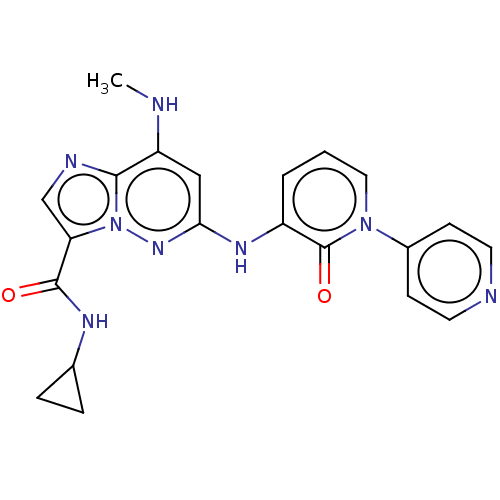 (CHEMBL4585272) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526608 (CHEMBL4547009) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526623 (CHEMBL4466139 | US11174264, Compound I-4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526618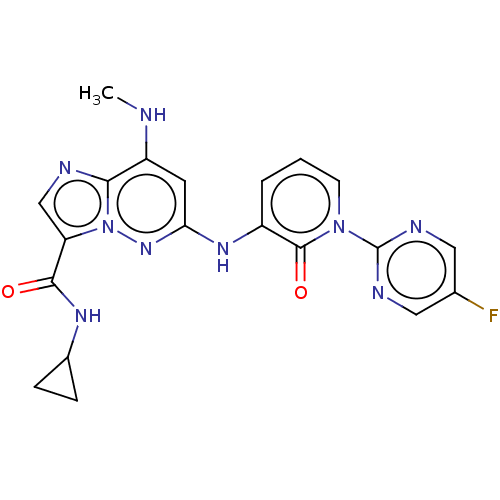 (CHEMBL4437714) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526609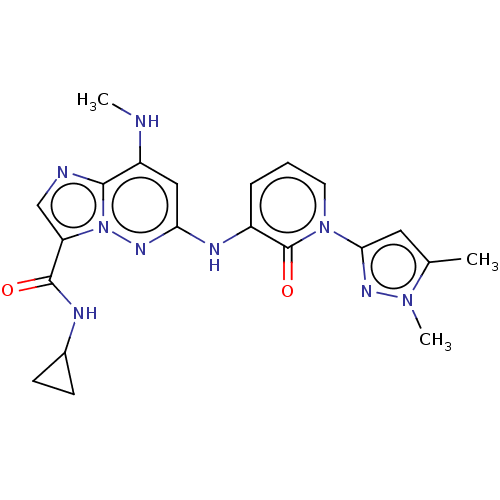 (CHEMBL4454109) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526614 (CHEMBL4517542) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19202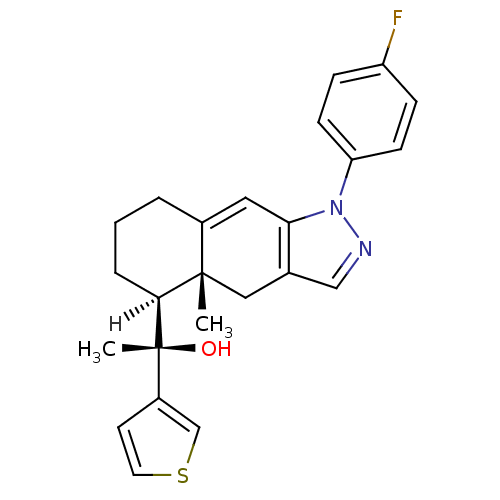 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582798 (CHEMBL5082323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303827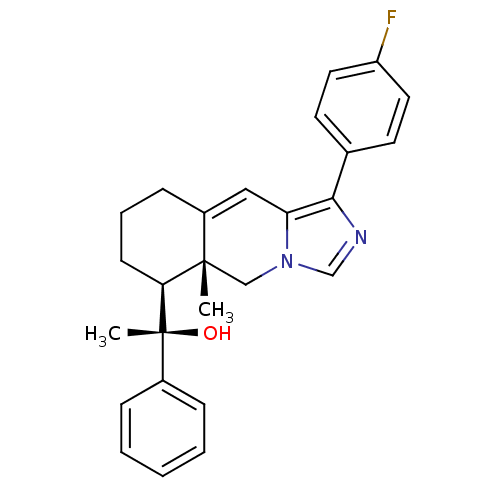 ((S)-1-((5aR,6S)-1-(4-fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303831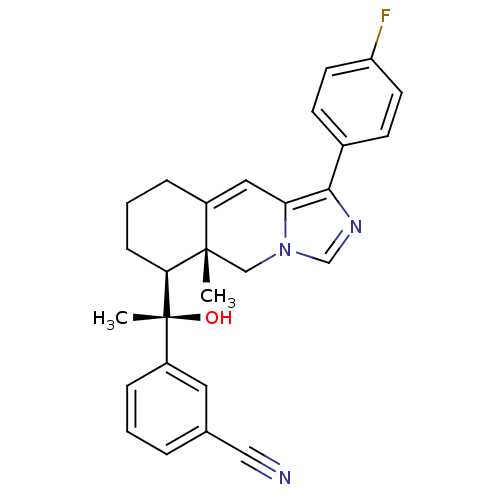 (3-((S)-1-((5aR,6S)-1-(4-fluorophenyl)-5a-methyl-5,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440488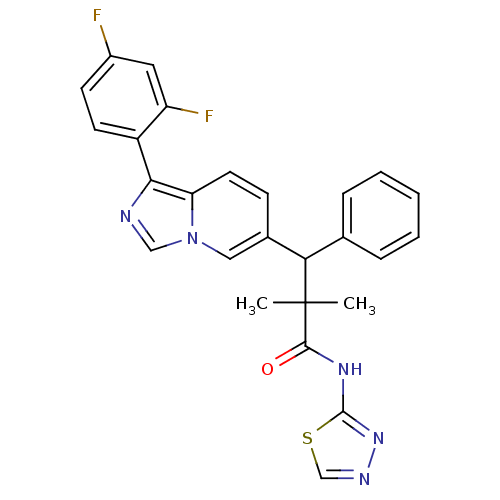 (CHEMBL2426147) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5571-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.049 BindingDB Entry DOI: 10.7270/Q2QC04Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303813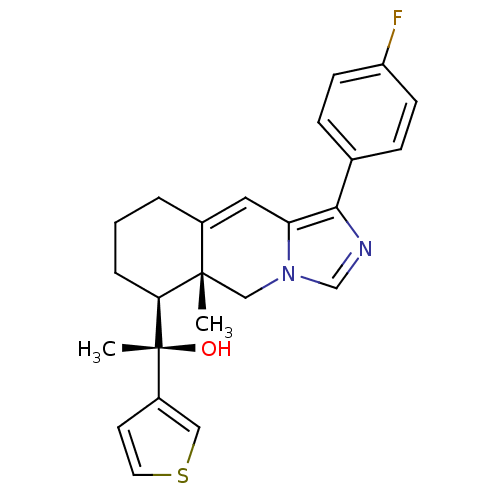 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5571-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.049 BindingDB Entry DOI: 10.7270/Q2QC04Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582795 (CHEMBL5081455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582794 (CHEMBL5077709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303811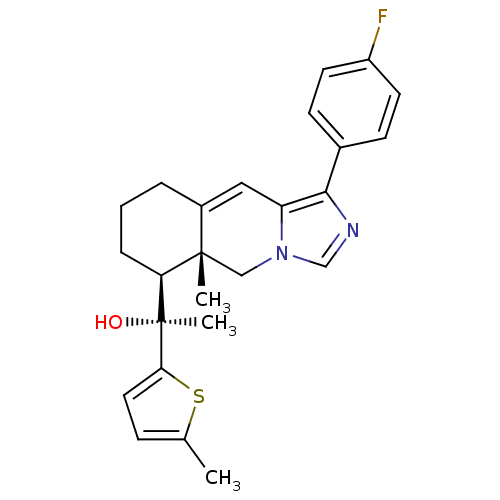 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582793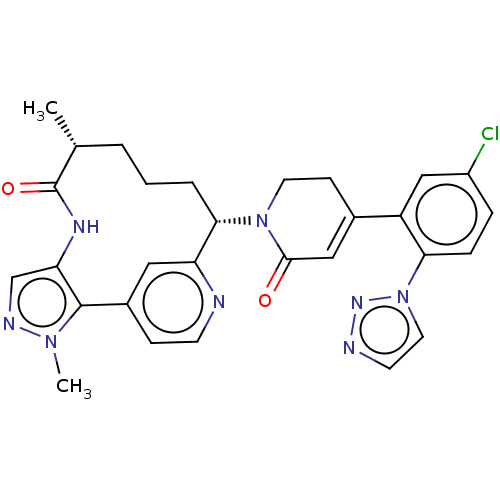 (CHEMBL5082956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19223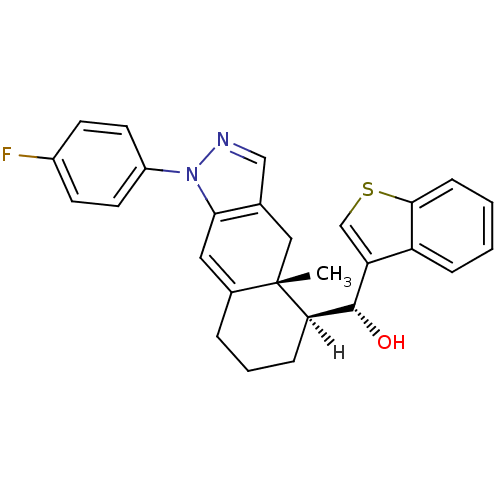 ((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303826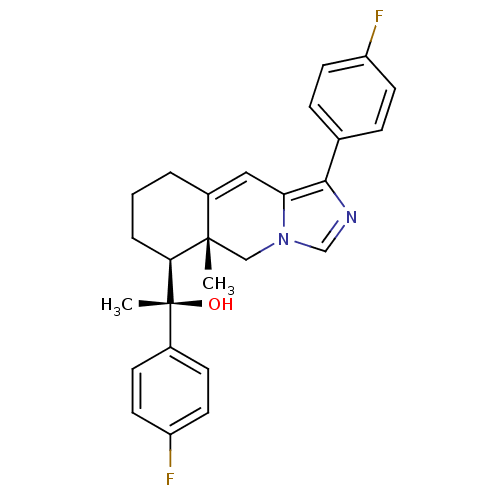 ((S)-1-(4-Fluorophenyl)-1-((5aR,6S)-1-(4-fluorophen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303828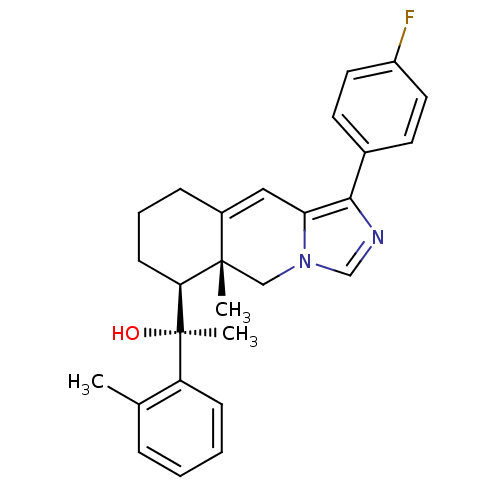 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303830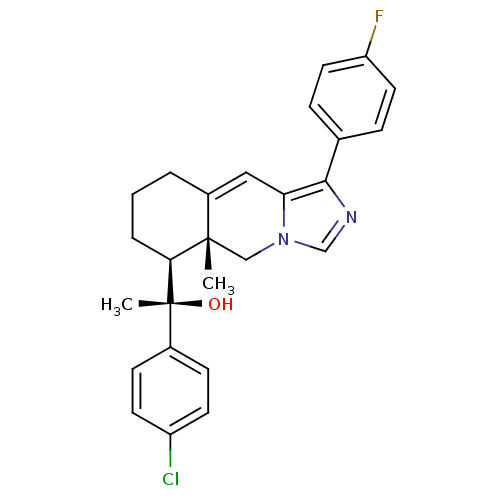 ((S)-1-(4-Chlorophenyl)-1-((5aR,6S)-1-(4-fluorophen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303808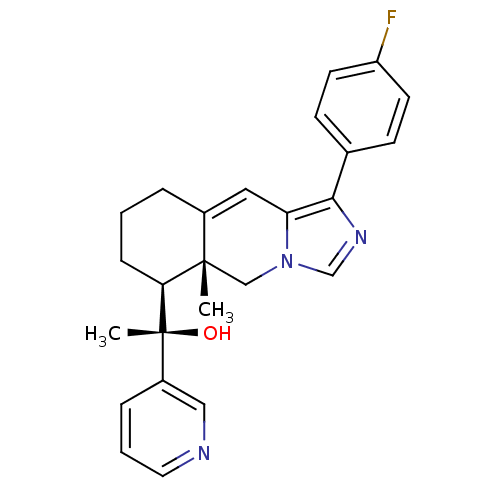 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303809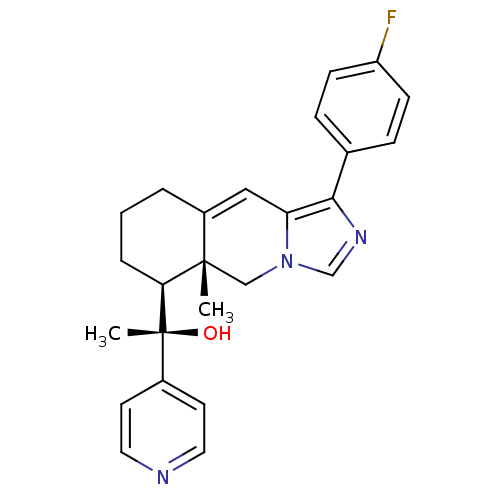 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303810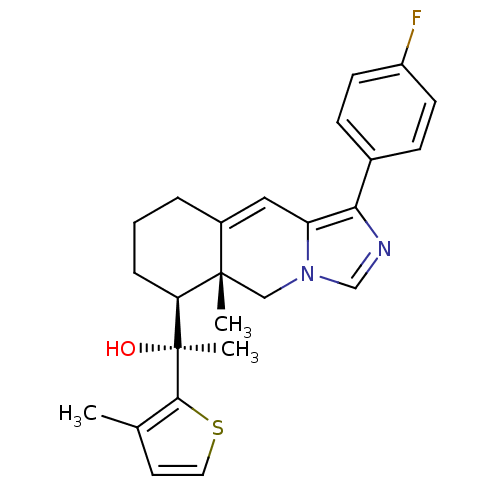 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303821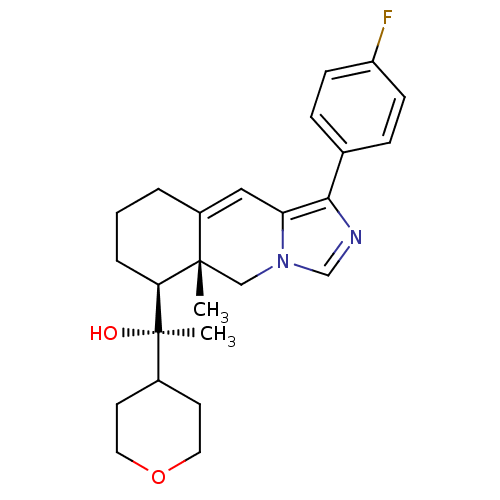 ((R)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440490 (CHEMBL2426146) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5571-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.049 BindingDB Entry DOI: 10.7270/Q2QC04Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440501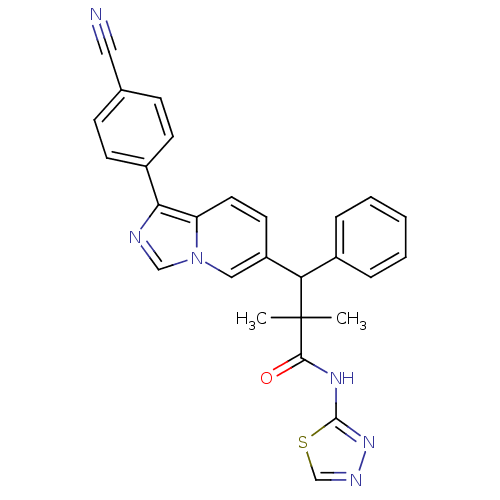 (CHEMBL2426144) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5571-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.049 BindingDB Entry DOI: 10.7270/Q2QC04Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303824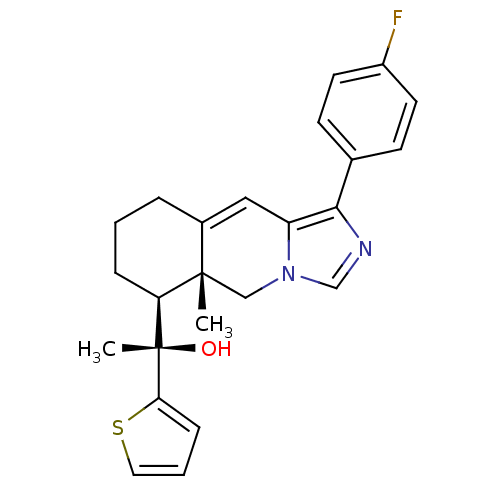 ((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2478 total ) | Next | Last >> |