 Found 17844 hits with Last Name = 'yan' and Initial = 'c'
Found 17844 hits with Last Name = 'yan' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253328
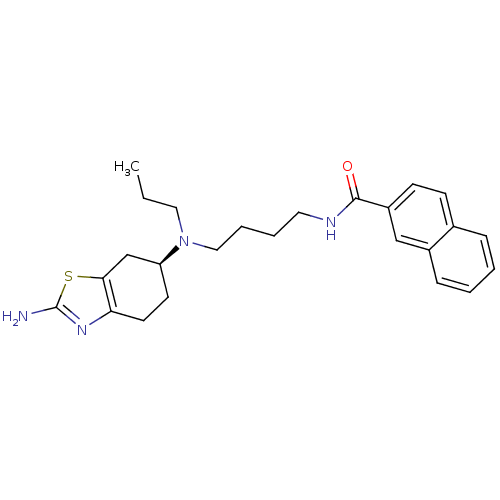
((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...)Show SMILES CCCN(CCCCNC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r| Show InChI InChI=1S/C25H32N4OS/c1-2-14-29(21-11-12-22-23(17-21)31-25(26)28-22)15-6-5-13-27-24(30)20-10-9-18-7-3-4-8-19(18)16-20/h3-4,7-10,16,21H,2,5-6,11-15,17H2,1H3,(H2,26,28)(H,27,30)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
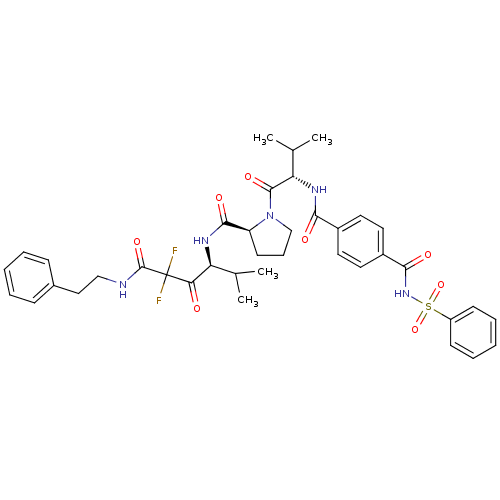
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50029246
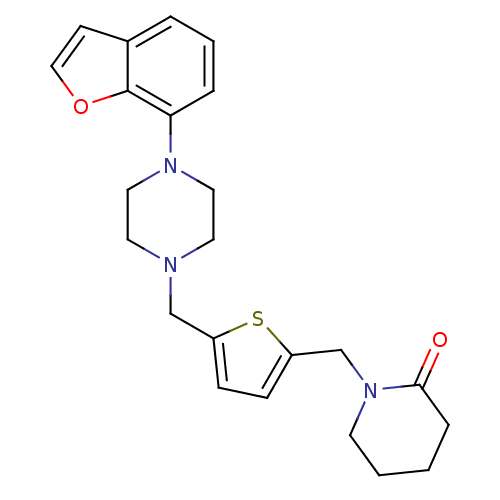
(1-[5-(4-Benzofuran-7-yl-piperazin-1-ylmethyl)-thio...)Show SMILES O=C1CCCCN1Cc1ccc(CN2CCN(CC2)c2cccc3ccoc23)s1 Show InChI InChI=1S/C23H27N3O2S/c27-22-6-1-2-10-26(22)17-20-8-7-19(29-20)16-24-11-13-25(14-12-24)21-5-3-4-18-9-15-28-23(18)21/h3-5,7-9,15H,1-2,6,10-14,16-17H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined against 5-hydroxytryptamine 1A receptor using [3H]WB-4101 |
J Med Chem 38: 4198-210 (1995)
BindingDB Entry DOI: 10.7270/Q2GX4C61 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass... |
ACS Med Chem Lett 7: 100-4 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00428
BindingDB Entry DOI: 10.7270/Q25T3NCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344100
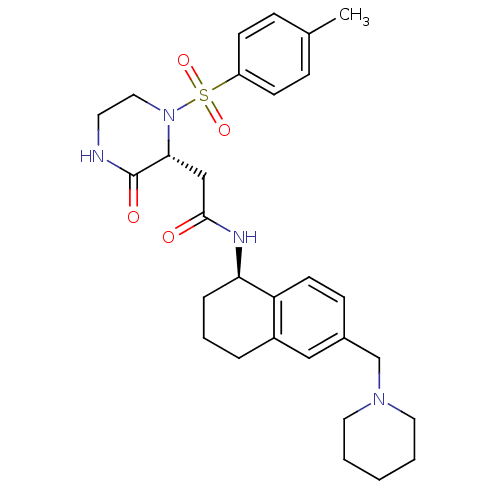
(2-((2R)-1-((4-methylphenyl)sulfonyl)-3-oxo-2-piper...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human B1 receptor |
J Med Chem 54: 7232-46 (2011)
Article DOI: 10.1021/jm200808v
BindingDB Entry DOI: 10.7270/Q28C9WP1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50391386
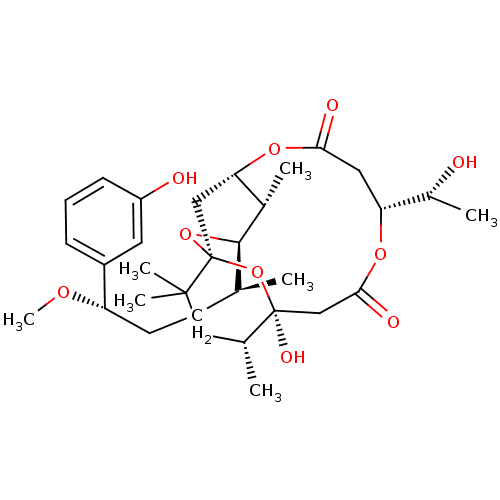
(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCeta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50327943
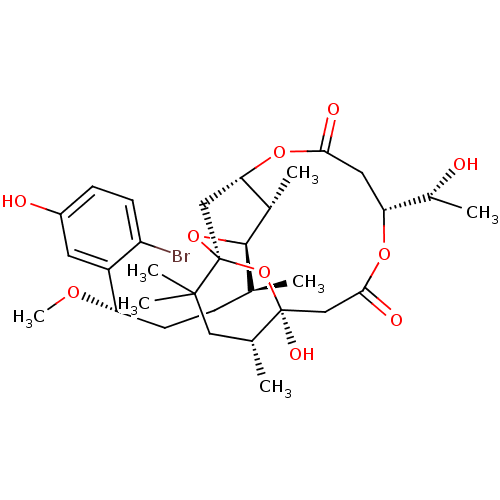
(Aplysiatoxin | CHEMBL1256416)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cc(O)ccc1Br |r| Show InChI InChI=1S/C32H47BrO10/c1-17(8-11-24(39-7)22-12-21(35)9-10-23(22)33)29-19(3)26-15-32(42-29)30(5,6)14-18(2)31(38,43-32)16-28(37)40-25(20(4)34)13-27(36)41-26/h9-10,12,17-20,24-26,29,34-35,38H,8,11,13-16H2,1-7H3/t17-,18+,19-,20+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCtheta C1B peptide |
Bioorg Med Chem Lett 20: 6064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.051
BindingDB Entry DOI: 10.7270/Q2FJ2H04 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504229

(N-[2-[(2R)-2-Fluoro-3-hydroxy- 3-methyl-butyl]-6-i...)Show SMILES CC(C)Oc1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cccnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50493217
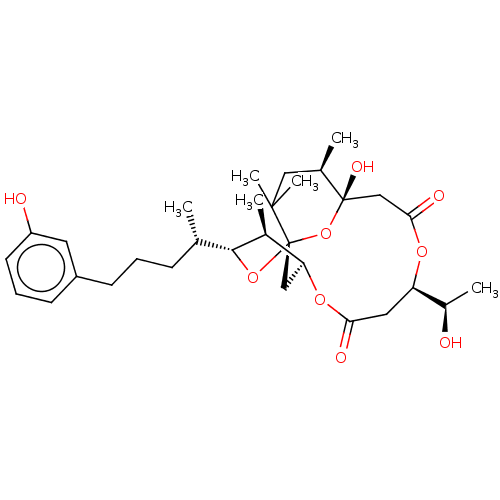
(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCeta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50459819
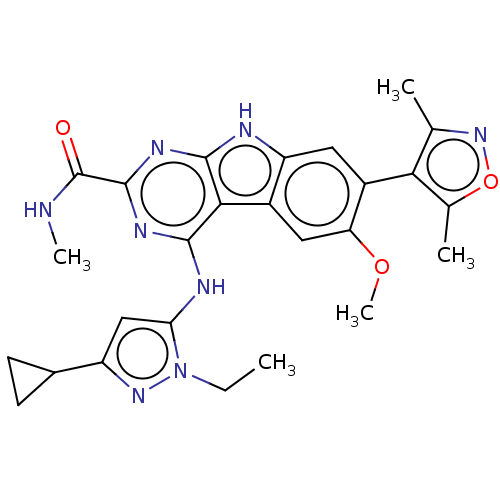
(CHEMBL4228445)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NC)C1CC1 |(34.96,-28.14,;33.49,-28.62,;32.34,-27.6,;32.49,-26.07,;31.08,-25.45,;30.06,-26.6,;30.85,-27.93,;30.23,-29.34,;31.14,-30.58,;32.66,-30.41,;33.58,-31.65,;32.96,-33.05,;31.44,-33.22,;30.55,-34.48,;29.08,-34.01,;27.74,-34.79,;26.4,-34.02,;25.07,-34.79,;24.91,-36.32,;26.05,-37.35,;23.4,-36.64,;22.63,-35.31,;23.67,-34.16,;23.35,-32.66,;26.4,-32.47,;25.07,-31.7,;25.07,-30.16,;27.73,-31.7,;29.07,-32.47,;30.53,-31.98,;35.11,-31.48,;35.73,-30.07,;36.02,-32.72,;35.4,-34.13,;30.74,-23.95,;31.21,-22.48,;29.7,-22.81,)| Show InChI InChI=1S/C26H28N8O3/c1-6-34-20(11-17(32-34)14-7-8-14)29-24-22-15-10-19(36-5)16(21-12(2)33-37-13(21)3)9-18(15)28-23(22)30-25(31-24)26(35)27-4/h9-11,14H,6-8H2,1-5H3,(H,27,35)(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 1 (24 to 144 residues) (unknown origin) expressed in ... |
J Med Chem 61: 462-481 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01816
BindingDB Entry DOI: 10.7270/Q2CV4MD0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181840
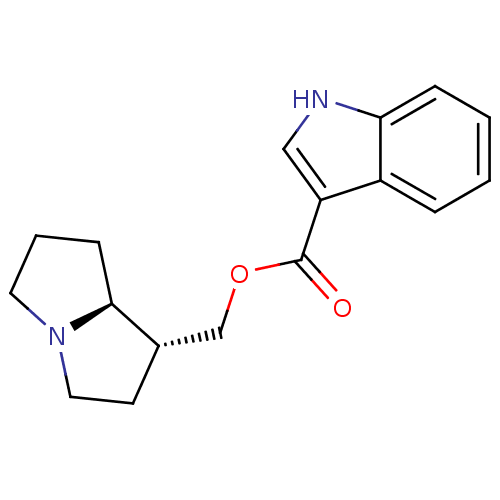
((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-Meth...)Show SMILES O=C(OC[C@@H]1CCN2CCC[C@@H]12)c1c[nH]c2ccccc12 Show InChI InChI=1S/C17H20N2O2/c20-17(14-10-18-15-5-2-1-4-13(14)15)21-11-12-7-9-19-8-3-6-16(12)19/h1-2,4-5,10,12,16,18H,3,6-9,11H2/t12-,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181844
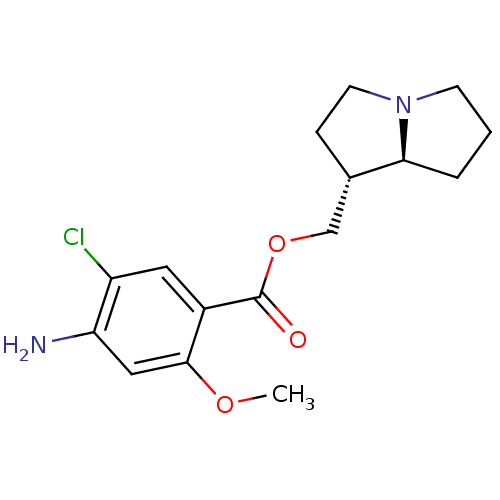
((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 4-amin...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H21ClN2O3/c1-21-15-8-13(18)12(17)7-11(15)16(20)22-9-10-4-6-19-5-2-3-14(10)19/h7-8,10,14H,2-6,9,18H2,1H3/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50029257

((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...)Show SMILES CC(C)Oc1ccccc1N1CCN(Cc2cccc(c2)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C26H35N3O2/c1-21(2)31-25-12-5-4-11-24(25)28-17-15-27(16-18-28)20-22-9-8-10-23(19-22)26(30)29-13-6-3-7-14-29/h4-5,8-12,19,21H,3,6-7,13-18,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha-1A adrenergic receptors using alpha1A ligand WB4101 |
J Med Chem 37: 1060-2 (1994)
BindingDB Entry DOI: 10.7270/Q23R0RXB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636
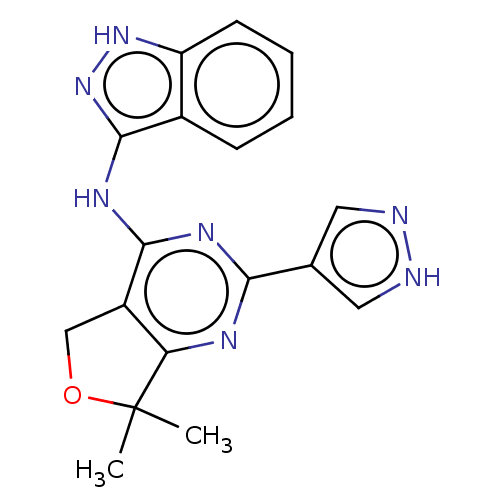
(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCtheta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCdelta C1B domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined against Dopamine receptor D2 using [3H]spiperone |
J Med Chem 38: 4198-210 (1995)
BindingDB Entry DOI: 10.7270/Q2GX4C61 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854
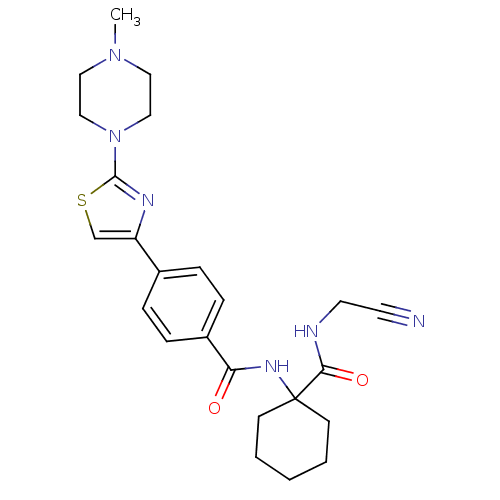
(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036095
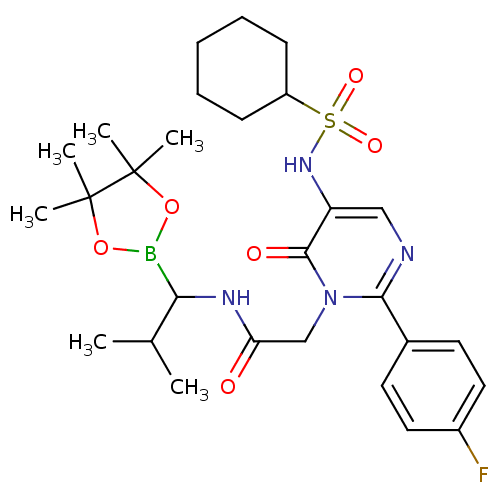
(2-[5-Cyclohexanesulfonylamino-2-(4-fluoro-phenyl)-...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(NS(=O)(=O)C2CCCCC2)c1=O)-c1ccc(F)cc1)B1OC(C)(C)C(C)(C)O1 Show InChI InChI=1S/C28H40BFN4O6S/c1-18(2)24(29-39-27(3,4)28(5,6)40-29)32-23(35)17-34-25(19-12-14-20(30)15-13-19)31-16-22(26(34)36)33-41(37,38)21-10-8-7-9-11-21/h12-16,18,21,24,33H,7-11,17H2,1-6H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of human leukocyte elastase mediated hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNA |
J Med Chem 38: 98-108 (1995)
BindingDB Entry DOI: 10.7270/Q2JW8CX7 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50459819

(CHEMBL4228445)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NC)C1CC1 |(34.96,-28.14,;33.49,-28.62,;32.34,-27.6,;32.49,-26.07,;31.08,-25.45,;30.06,-26.6,;30.85,-27.93,;30.23,-29.34,;31.14,-30.58,;32.66,-30.41,;33.58,-31.65,;32.96,-33.05,;31.44,-33.22,;30.55,-34.48,;29.08,-34.01,;27.74,-34.79,;26.4,-34.02,;25.07,-34.79,;24.91,-36.32,;26.05,-37.35,;23.4,-36.64,;22.63,-35.31,;23.67,-34.16,;23.35,-32.66,;26.4,-32.47,;25.07,-31.7,;25.07,-30.16,;27.73,-31.7,;29.07,-32.47,;30.53,-31.98,;35.11,-31.48,;35.73,-30.07,;36.02,-32.72,;35.4,-34.13,;30.74,-23.95,;31.21,-22.48,;29.7,-22.81,)| Show InChI InChI=1S/C26H28N8O3/c1-6-34-20(11-17(32-34)14-7-8-14)29-24-22-15-10-19(36-5)16(21-12(2)33-37-13(21)3)9-18(15)28-23(22)30-25(31-24)26(35)27-4/h9-11,14H,6-8H2,1-5H3,(H,27,35)(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 2 (306 to 417residues) (unknown origin) expressed in ... |
J Med Chem 61: 462-481 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01816
BindingDB Entry DOI: 10.7270/Q2CV4MD0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50064563
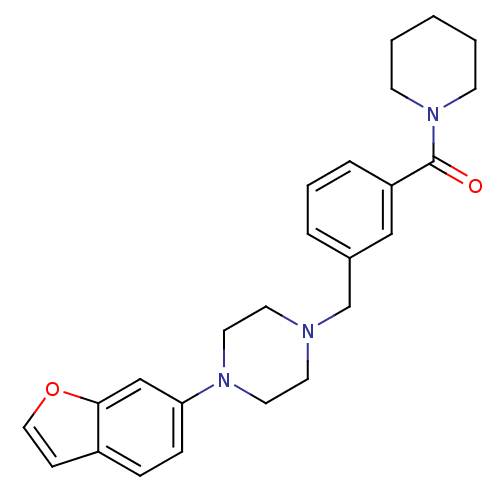
(CHEMBL61816 | [3-(4-Benzofuran-6-yl-piperazin-1-yl...)Show SMILES O=C(N1CCCCC1)c1cccc(CN2CCN(CC2)c2ccc3ccoc3c2)c1 Show InChI InChI=1S/C25H29N3O2/c29-25(28-10-2-1-3-11-28)22-6-4-5-20(17-22)19-26-12-14-27(15-13-26)23-8-7-21-9-16-30-24(21)18-23/h4-9,16-18H,1-3,10-15,19H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex |
J Med Chem 41: 1997-2009 (1998)
Article DOI: 10.1021/jm970164z
BindingDB Entry DOI: 10.7270/Q2KK9CG0 |
More data for this
Ligand-Target Pair | |
Apelin receptor
(Homo sapiens (Human)) | BDBM50556826

(CHEMBL4745863)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CCC(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01913
BindingDB Entry DOI: 10.7270/Q2BG2SNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50368604
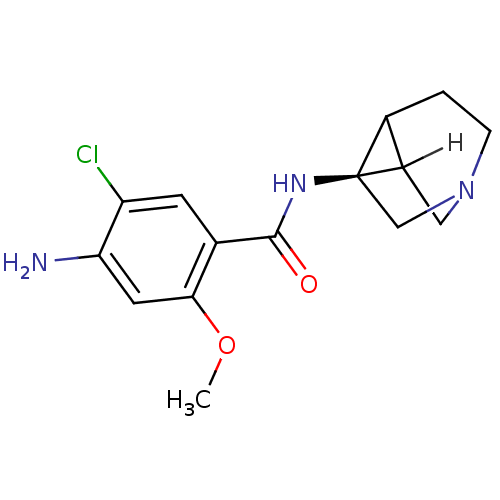
(CHEMBL1907770)Show SMILES [H]C12CN3C[C@]1(NC(=O)c1cc(Cl)c(N)cc1OC)C2CC3 |r| Show InChI InChI=1S/C15H18ClN3O2/c1-21-13-5-12(17)11(16)4-8(13)14(20)18-15-7-19-3-2-9(15)10(15)6-19/h4-5,9-10H,2-3,6-7,17H2,1H3,(H,18,20)/t9?,10?,15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor |
J Med Chem 35: 1486-9 (1992)
BindingDB Entry DOI: 10.7270/Q2CC11B3 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50255183
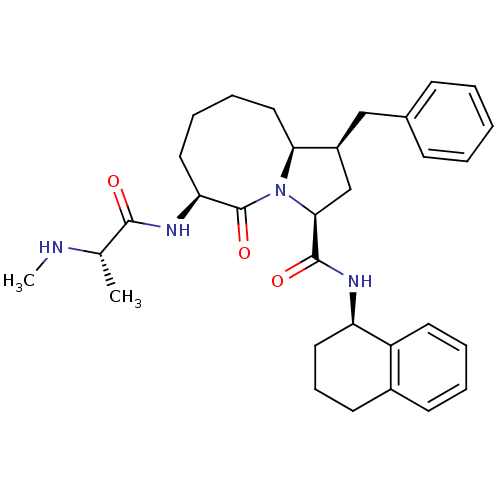
((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCCC[C@H]2[C@@H](Cc3ccccc3)C[C@H](N2C1=O)C(=O)N[C@@H]1CCCc2ccccc12 |r| Show InChI InChI=1S/C32H42N4O3/c1-21(33-2)30(37)35-27-16-8-9-18-28-24(19-22-11-4-3-5-12-22)20-29(36(28)32(27)39)31(38)34-26-17-10-14-23-13-6-7-15-25(23)26/h3-7,11-13,15,21,24,26-29,33H,8-10,14,16-20H2,1-2H3,(H,34,38)(H,35,37)/t21-,24-,26+,27-,28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of fluorescent SM5F peptide from His-tagged human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... |
J Med Chem 52: 593-6 (2009)
Article DOI: 10.1021/jm801101z
BindingDB Entry DOI: 10.7270/Q2Z03816 |
More data for this
Ligand-Target Pair | |
Apelin receptor
(Homo sapiens (Human)) | BDBM50556827

(CHEMBL4748838)Show SMILES CSCC[C@H](NC(=O)[C@H](CS)NC(=O)CC[C@H](NC(=O)CCOCCOCCOCCOCCOCCOCCN)C(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01913
BindingDB Entry DOI: 10.7270/Q2BG2SNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50029262
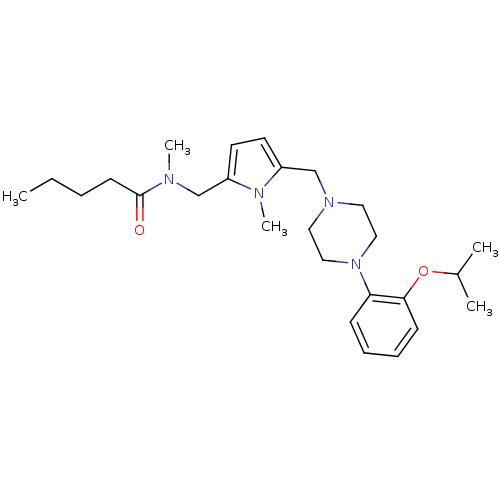
(Pentanoic acid {5-[4-(2-isopropoxy-phenyl)-piperaz...)Show SMILES CCCCC(=O)N(C)Cc1ccc(CN2CCN(CC2)c2ccccc2OC(C)C)n1C Show InChI InChI=1S/C26H40N4O2/c1-6-7-12-26(31)27(4)19-22-13-14-23(28(22)5)20-29-15-17-30(18-16-29)24-10-8-9-11-25(24)32-21(2)3/h8-11,13-14,21H,6-7,12,15-20H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity was determined against 5-hydroxytryptamine 1A receptor using [3H]WB-4101 |
J Med Chem 38: 4198-210 (1995)
BindingDB Entry DOI: 10.7270/Q2GX4C61 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
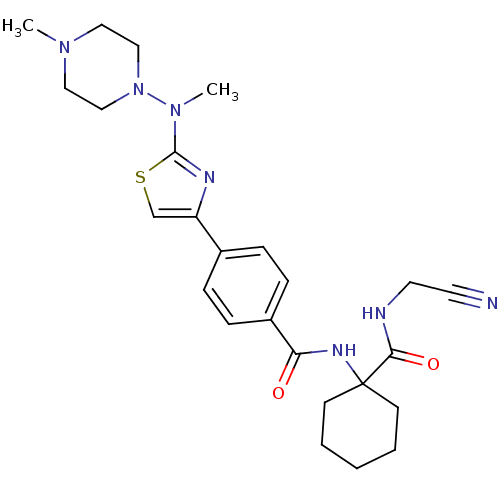
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504219
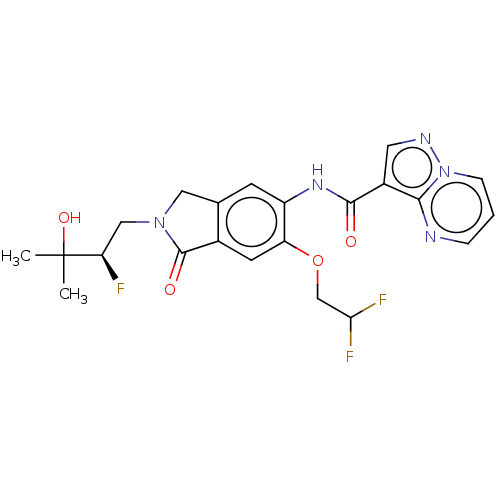
((R)-N-(6-(2,2-Difluoroethoxy)- 2-(2-fluoro-3-hydro...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OCC(F)F)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCalpha C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50459819

(CHEMBL4228445)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NC)C1CC1 |(34.96,-28.14,;33.49,-28.62,;32.34,-27.6,;32.49,-26.07,;31.08,-25.45,;30.06,-26.6,;30.85,-27.93,;30.23,-29.34,;31.14,-30.58,;32.66,-30.41,;33.58,-31.65,;32.96,-33.05,;31.44,-33.22,;30.55,-34.48,;29.08,-34.01,;27.74,-34.79,;26.4,-34.02,;25.07,-34.79,;24.91,-36.32,;26.05,-37.35,;23.4,-36.64,;22.63,-35.31,;23.67,-34.16,;23.35,-32.66,;26.4,-32.47,;25.07,-31.7,;25.07,-30.16,;27.73,-31.7,;29.07,-32.47,;30.53,-31.98,;35.11,-31.48,;35.73,-30.07,;36.02,-32.72,;35.4,-34.13,;30.74,-23.95,;31.21,-22.48,;29.7,-22.81,)| Show InChI InChI=1S/C26H28N8O3/c1-6-34-20(11-17(32-34)14-7-8-14)29-24-22-15-10-19(36-5)16(21-12(2)33-37-13(21)3)9-18(15)28-23(22)30-25(31-24)26(35)27-4/h9-11,14H,6-8H2,1-5H3,(H,27,35)(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD2 bromodomain 2 (349 to 460 residues) (unknown origin) expressed in... |
J Med Chem 61: 462-481 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01816
BindingDB Entry DOI: 10.7270/Q2CV4MD0 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504215

(N-[6-(3-Fluorocyclobutoxy)-2- [(2R)-2-fluoro-3-hyd...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@@H]3C[C@H](F)C3)cc2C1=O |r,wU:26.27,28.30,wD:4.4,(-7.2,.34,;-7.24,1.88,;-8.73,1.48,;-8.01,3.22,;-5.7,1.88,;-4.93,3.22,;-4.93,.55,;-3.39,.55,;-2.49,1.79,;-1.02,1.32,;.31,2.09,;1.64,1.32,;2.98,2.09,;2.98,3.63,;1.64,4.4,;4.31,4.4,;4.47,5.93,;5.98,6.25,;6.75,4.92,;8.25,4.6,;8.73,3.13,;7.7,1.99,;6.19,2.31,;5.72,3.77,;1.64,-.22,;2.98,-.99,;2.98,-2.53,;1.89,-3.62,;2.98,-4.71,;2.98,-6.25,;4.07,-3.62,;.31,-.99,;-1.02,-.22,;-2.49,-.7,;-2.96,-2.16,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
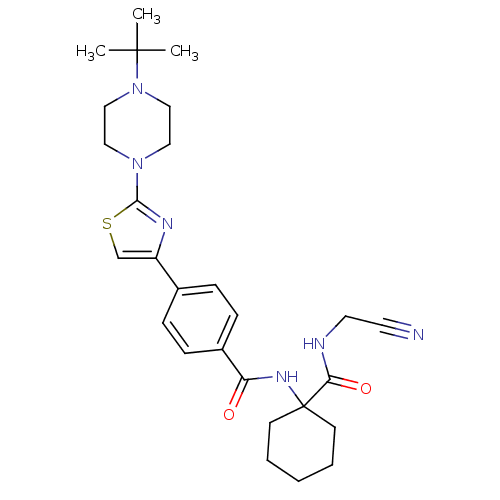
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315206
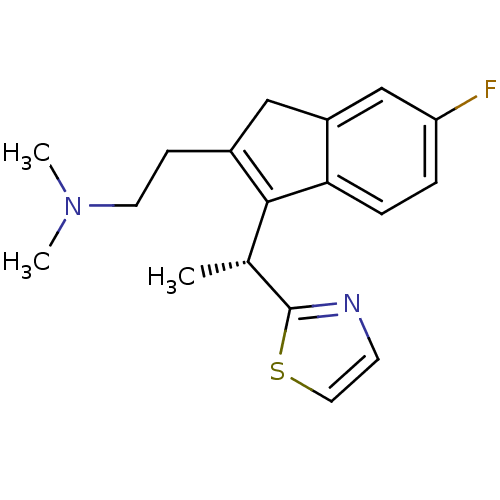
((R)-2-(6-fluoro-3-(1-(thiazol-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2cc(F)ccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C18H21FN2S/c1-12(18-20-7-9-22-18)17-13(6-8-21(2)3)10-14-11-15(19)4-5-16(14)17/h4-5,7,9,11-12H,6,8,10H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559603

(CHEMBL4787458)Show SMILES Cc1cc(OC2CCC3(CCCC3)CC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human S1P5 receptor expressed in Chem-1 cell membrane by 33P-SIP binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075926
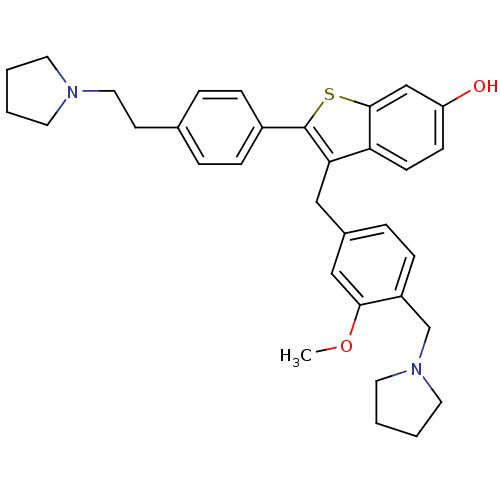
(3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(CCN3CCCC3)cc2)ccc1CN1CCCC1 Show InChI InChI=1S/C33H38N2O2S/c1-37-31-21-25(8-11-27(31)23-35-17-4-5-18-35)20-30-29-13-12-28(36)22-32(29)38-33(30)26-9-6-24(7-10-26)14-19-34-15-2-3-16-34/h6-13,21-22,36H,2-5,14-20,23H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50355063
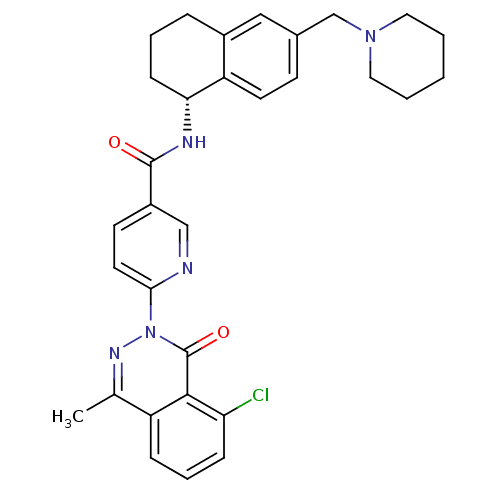
(CHEMBL1834619)Show SMILES Cc1nn(-c2ccc(cn2)C(=O)N[C@@H]2CCCc3cc(CN4CCCCC4)ccc23)c(=O)c2c(Cl)cccc12 |r| Show InChI InChI=1S/C31H32ClN5O2/c1-20-24-8-6-9-26(32)29(24)31(39)37(35-20)28-14-12-23(18-33-28)30(38)34-27-10-5-7-22-17-21(11-13-25(22)27)19-36-15-3-2-4-16-36/h6,8-9,11-14,17-18,27H,2-5,7,10,15-16,19H2,1H3,(H,34,38)/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human B1 receptor |
J Med Chem 54: 7232-46 (2011)
Article DOI: 10.1021/jm200808v
BindingDB Entry DOI: 10.7270/Q28C9WP1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCdelta C1B domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50064537

(CHEMBL293658 | {3-[4-(2,3-Dihydro-benzo[1,4]dioxin...)Show SMILES O=C(N1CCCCC1)c1cccc(CN2CCN(CC2)C2COc3ccccc3O2)c1 Show InChI InChI=1S/C25H31N3O3/c29-25(28-11-4-1-5-12-28)21-8-6-7-20(17-21)18-26-13-15-27(16-14-26)24-19-30-22-9-2-3-10-23(22)31-24/h2-3,6-10,17,24H,1,4-5,11-16,18-19H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex |
J Med Chem 41: 1997-2009 (1998)
Article DOI: 10.1021/jm970164z
BindingDB Entry DOI: 10.7270/Q2KK9CG0 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50229772
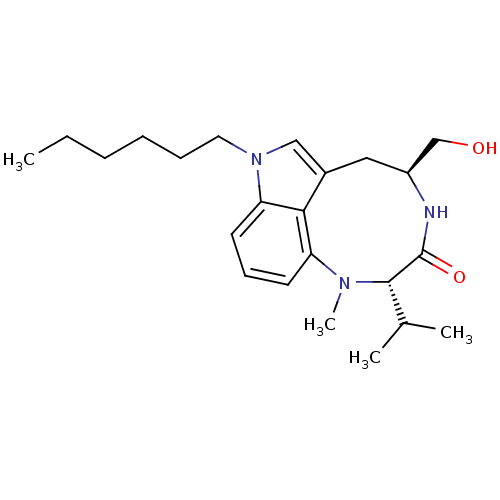
(1-hexylindolactam-V | CHEMBL399981)Show SMILES CCCCCCn1cc2C[C@@H](CO)NC(=O)[C@H](C(C)C)N(C)c3cccc1c23 Show InChI InChI=1S/C23H35N3O2/c1-5-6-7-8-12-26-14-17-13-18(15-27)24-23(28)22(16(2)3)25(4)19-10-9-11-20(26)21(17)19/h9-11,14,16,18,22,27H,5-8,12-13,15H2,1-4H3,(H,24,28)/t18-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCeta C1B domain |
J Med Chem 51: 46-56 (2008)
Article DOI: 10.1021/jm0706719
BindingDB Entry DOI: 10.7270/Q2445M7Q |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061028
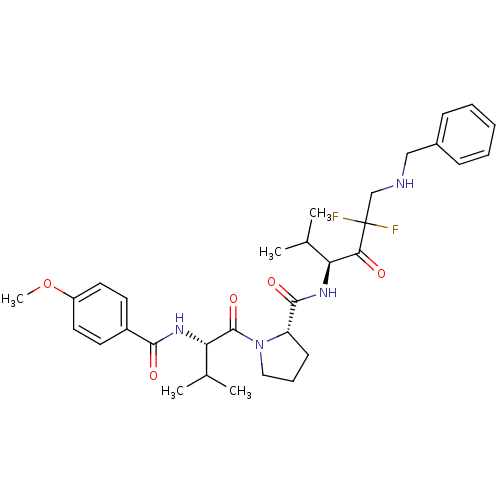
((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...)Show SMILES COc1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNCc1ccccc1 Show InChI InChI=1S/C32H42F2N4O5/c1-20(2)26(28(39)32(33,34)19-35-18-22-10-7-6-8-11-22)36-30(41)25-12-9-17-38(25)31(42)27(21(3)4)37-29(40)23-13-15-24(43-5)16-14-23/h6-8,10-11,13-16,20-21,25-27,35H,9,12,17-19H2,1-5H3,(H,36,41)(H,37,40)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061043
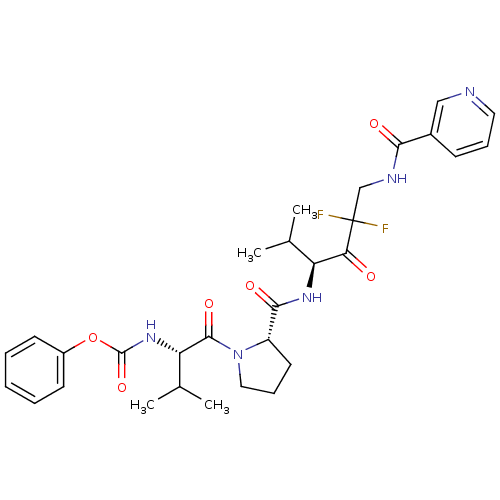
(CHEMBL106592 | [(S)-1-((S)-2-{(S)-3,3-Difluoro-1-i...)Show SMILES CC(C)[C@H](NC(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNC(=O)c1cccnc1 Show InChI InChI=1S/C30H37F2N5O6/c1-18(2)23(25(38)30(31,32)17-34-26(39)20-10-8-14-33-16-20)35-27(40)22-13-9-15-37(22)28(41)24(19(3)4)36-29(42)43-21-11-6-5-7-12-21/h5-8,10-12,14,16,18-19,22-24H,9,13,15,17H2,1-4H3,(H,34,39)(H,35,40)(H,36,42)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695
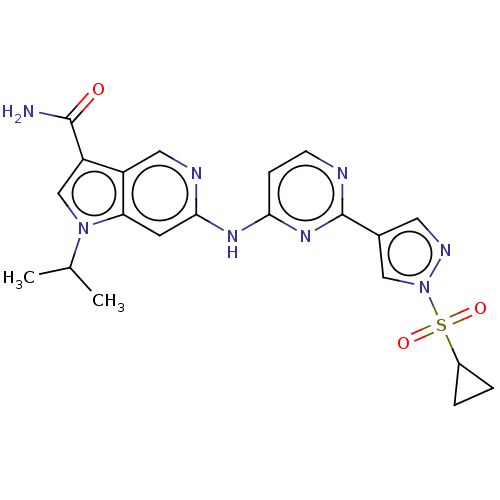
(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data